Qingshan He a 1, Jiaran Zhu a 1, Guojun Yang b 1, Xiufei Liu a 1, Lu Li a, Yuren Wang a, Xin Xiong a, Yi Zheng a, Hongting Zheng a, Hua Qu a
Abstract
Background
Diabetic cardiomyopathy (DbCM) is defined as the existence of abnormal myocardial structure and functions in the absence of other cardiac diseases, such as coronary artery disease, hypertension, and significant valvular disease, in individuals with diabetes. Although abundant epidemic evidence demonstrates that diabetes is independently associated with the risk of developing heart failure, DbCM is not normally diagnosed in clinical practices due to its exclusive diagnosis, and no diagnostic biomarker was applied in a clinical test.
Methods
To detect the concentrations of serum Annexin A2 in non-diabetic subjects, type 2 diabetic (T2DM) patients with or without DbCM, and analyzed its relationship to parameters of cardiac functions, glucose, lipid metabolism, and renal functions. 266 eligible participants were included and were divided into 3 groups including non-diabetic subjects (NGR), T2DM patients without DbCM (T2DM group), and the DbCM group. Echocardiography, coronary computed tomography angiography, electrocardiogram, blood pressure, thyroid function, and clinical and other biochemical parameters were measured in all participants.
Results
Serum Annexin A2 concentrations were higher in DbCM (P < 0.05) and T2DM (P < 0.05) groups compared with the NGR group, especially in DbCM patients. Correlation analysis showed that serum Annexin A2 levels were negatively associated with left ventricular (LV) ejection fraction (EF), LV fractional shortening (FS), the ratio of early (E-wave) and late (A-wave) LV diastolic filling velocities (E/A ratio), and estimated glomerular filtration rate (eGFR), and were positively correlated with age, blood urea nitrogen (BUN) and creatinine (Cr) (all P < 0.05). Multiple logistical regression analyses revealed that serum in both the second and the third tertiles of Annexin A2 concentration were significantly associated with DbCM. E/A ratio is the independent factor for Annexin A2 concentration when adjusted for LV FS%, BUN, and Cr.
Conclusions
Circulating Annexin A2 concentrations might be induced in DbCM patients and were negatively associated with cardiac systolic and diastolic functions, which suggested it might be a predictor of early diagnosis in DbCM and might be a potential therapeutic target for DbCM.
Keywords
Annexin A2; Diabetes Cardiac function; Diabetic cardiomyopathy
1. Introduction
Since 1972 Rubler et al. 1 reported several diabetic patients with cardiac structure and function disorders presented in diabetic patients cannot be explained by coronary artery disease, valvular or congenital heart disease, hypertension, or alcoholism, diabetic cardiomyopathy (DbCM) has now been accepted as a chronic complication of diabetes independent of other known cardiac diseases 2. DbCM is defined as the existence of abnormal cardiac structure and performance in the absence of other cardiac risk factors, such as coronary artery disease, hypertension, and significant valvular disease 3,4. Clinically, DbCM is characterized by cardiac functional and structural alterations such as left ventricular (LV) diastolic and systolic dysfunction, increases in interstitial and perivascular fibrosis, and LV hypertrophy 3. However, no specific biochemical markers or clinical manifestations were included in the diagnosis of DbCM. It is often asymptomatic in the early stage and usually overlaps with other diabetic complications during the progression, making a definitive diagnosis challenging 3. Therefore, early diagnostic markers are urgent to be identified.
Annexin A2 belongs to the family of Annexins, and as a calcium-dependent phospholipid-binding protein, this family plays a role in the regulation of cellular growth and signal transduction pathways 5,6. As one of the most abundant cardiac Annexins (Annexin A2, A5, and A6 are particularly abundant in myocardial tissue) 7, A2 is involved in excitation–contraction coupling or electromechanical coupling, as well as in interactions with membrane proteins like ion channels or transporters, these functions were deeply associated with cardiac function 6. Indeed, previous studies in hypertrophied LV from hypertensive guinea pigs and failing hearts from humans revealed that the level of Annexin A2 was significantly increased in hypertrophied and failing hearts 8, 9. Furthermore, serum Annexin A2 concentrations were also reported to increase in several diabetic microvascular complications, including diabetic nephropathy and diabetic retinopathy 10,11. Considering DbCM is also recognized as a microvascular complication of diabetes, we speculate that its circulating levels might also be altered in DbCM and associated with cardiac functions.
To demonstrate this speculation, the current study recruited a total of 266 subjects and divided them into 3 groups including non-diabetic subjects (NGR group), T2DM patients without DbCM (T2DM group) and the DbCM group. All participants were screened by echocardiography, coronary computed tomography angiography, electrocardiogram, blood pressure, thyroid function, and clinical and other biochemical parameters. To detect the concentrations of serum Annexin A2 in 3 different groups and analyse its relationship to parameters of cardiac functions, glucose and lipid metabolism, and renal functions.
2. Methods
2.1. Study subjects
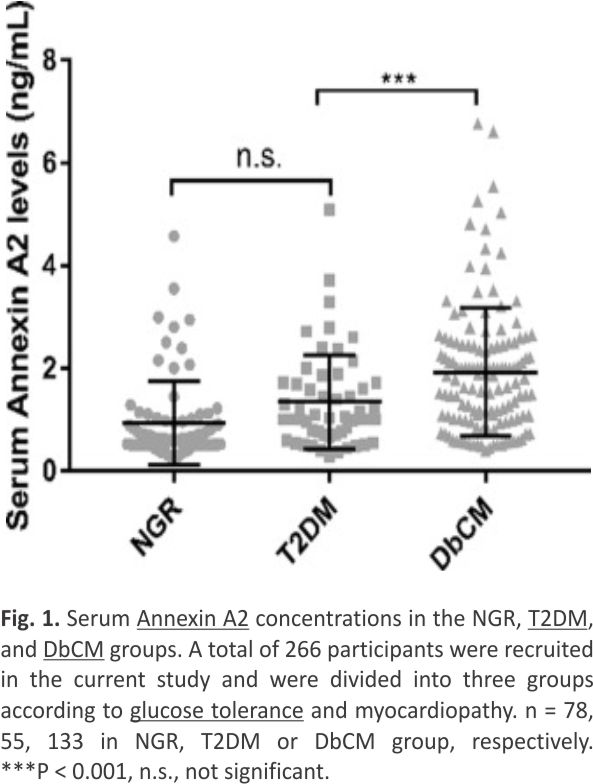
A total of 266 Chinese subjects aged 18 to 70 years were recruited for our study. Diabetes (DM) was confirmed according to the diagnostic criteria of WHO in 1998, that is, for DM, fasting plasma glucose (FPG) value ≥ 7.0 mmol/L or 2-h post glucose challenge (2hPG) ≥ 11.1 mmol/L or both 12. DbCM was diagnosed according to the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines 13. In general, T2DM patients with evidence of LV diastolic dysfunction and without other cardiac diseases, such as coronary heart disease, hypertensive heart disease, and dilated cardiomyopathy, were identified as DbCM. Then, the participants were divided into three groups: NGR (n = 78), T2DM (n = 55), and DbCM (n = 133) group.
The exclusion criteria included: (a) all patients were scanned for coronary computed tomography angiography (CTA) and subjects with coronary heart disease were excluded, (b) patients were diagnosed with hypertension, or any other cardiovascular diseases such as valvular diseases, arrhythmia, dilated or hyperthyroid cardiomyopathy; (c) history of other comorbidities, including tumor, severity hepatic and renal dysfunction, or significant psychiatric diseases; (d) suffering acute and serious chronic complications of diabetes; (e) have acute and chronic inflammatory diseases defined as increased C-reactive protein level or white blood cell count; (f) women who were currently pregnant were also excluded from this study. The sample size was calculated using PASS software (version 15.0; NCSS, Silver Spring, Md) and both the means of groups and the variance were set according to the results of our previous study 14. The power was set at 0.8, and the significance level was set at 0.05. All experimental protocols were approved by the Ethics Committee of Xinqiao Hospital, Third Military Medical University (Institutional Review Board–approved protocol number 2016–056-01). Written informed consent was collected for all subjects.
2.2. Clinical and biochemical evaluations
The height, body weight, and blood pressure were measured using standard protocols in all subjects. The body mass index (BMI) was calculated as the ratio of weight and squared height. Peripheral venous blood samples were collected in the morning after 8-hour overnight fasting. Plasma samples were obtained by centrifugation at 1,000 g for 15 min at 4 °C and kept at −80oC until used, within 3 months. Blood glucose and FPG were assayed using the glucose oxidase method. Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (VARIANTTM II and D-10TM Systems, BIO-RAD, USA). Lipid profiles, blood urea nitrogen (BUN), serum creatinine (Cr), uric acid (UA), and other liver and kidney function indicators and lipid profiles were detected by biochemical autoanalyzer (Beckman CX-7 Biochemical Autoanalyser, Brea, CA, USA). eGFR was estimated using the Modification of Diet in Renal Disease (MDRD) Study group equation 15.
2.3. Echocardiography and data calculations
Echocardiography was performed using a Vivid E9 ultrasound system (GE Healthcare, Milwaukee, WI, USA), and the main parameters assessed included: the LV end-diastolic diameter, LV end-systolic diameter, interventricular septal diameter, left ventricular posterior wall thickness, aortic diameter, LV EF%, FS%, peak early diastolic trans-mitral flow velocity (E), peak late diastolic trans-mitral flow velocity (A), mitral valve velocity (e), and the E/A ratio. Relative wall thickness (RWT) was calculated by the formula [(2 × diastolic posterior wall thickness)/diastolic LV internal diameter].
2.4. Assessment of serum Annexin A2 concentrations levels
Serum Annexin A2 concentrations Levels were determined by a commercial enzyme-linked immunosorbent kit according to the manufacturer’s instructions (Human ELISA kit, Shanghai Enzyme-linked Biotechnology Co). The intra-assay and inter-assay coefficients of variation were all less than 10%. All the assays were performed in duplicate and repeated if there was a > 15% difference between duplicates. No significant cross-reactivity or interference was observed.
2.5. Statistical analyses
All statistical analyses were conducted by SPSS software (IBM, Armonk, NY, version 19.0). Data were presented as mean values ± standard deviation (SD). Normal distribution of the data was detected using the Kolmogorov-Smirnov test. Several variables showed skewed distribution and were logarithmically transformed into normal distribution before statistical analysis. Analysis of variance (ANOVA) was performed for group comparisons. Interrelationships between variables were estimated using the Spearman correlation coefficient. Multivariate logistic regression analyses were used to analyze the association between Annexin A2 and DbCM. P values < 0.05 were regarded as statistically significant.
3. Results
3.1. The clinical characteristics in 3 different groups.
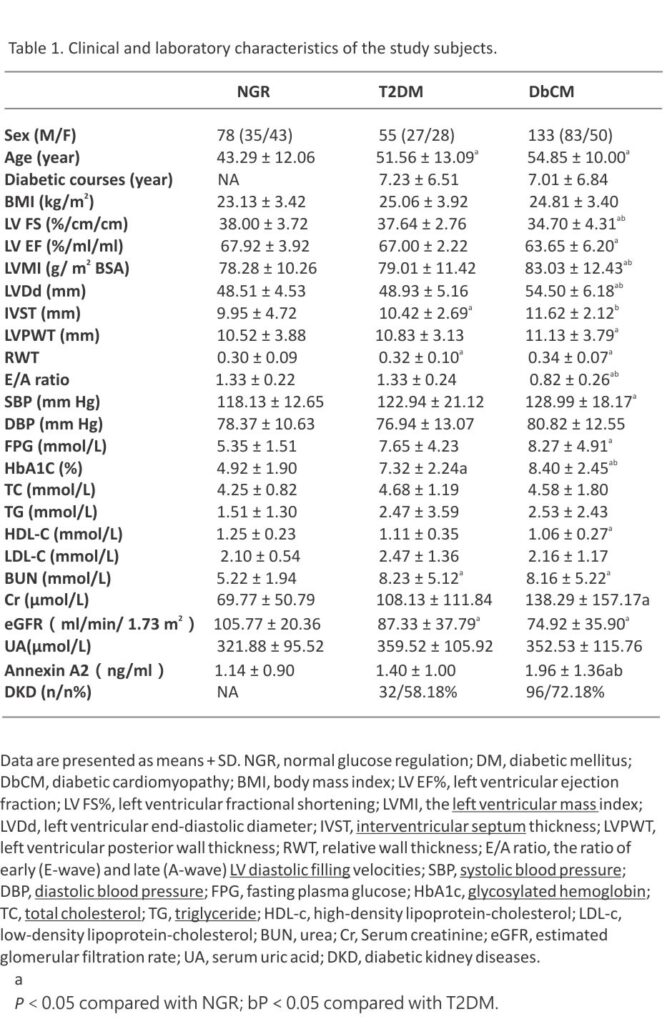
The main clinical characteristics of the subjects in different groups are shown in Table 1. Ages were highest in the DbCM group, second by the T2DM and NGR groups. Similarly, SBP, FPG, HbA1c, Cr, LVMI, LVDd, IVST, IVPWT, and RWT were highest in the DbCM group and then followed by T2DM and NGR groups. While, LV EF%, FS%, EA, HDL-c, and eGFR were lowest in the DbCM group, and higher in T2DM and NGR groups. In contrast with the NGR group, BUN was significantly increased and eGFR was decreased in the T2DM and DbCM groups, and Cr was increased in the DbCM group. BMI, DBP, TG, TC, LDL-c, and UA were not statistically different among the 3 groups. Moreover, the percentage of patients with DKD showed a higher trend in the DbCM group compared with the T2DM group (P = 0.061).
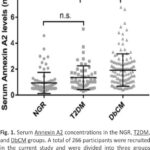
3.2. Serum Annexin A2 in these groups.
Compared to the NGR group, subjects displayed an increased trend of serum Annexin A2 levels in both T2DM and DbCM groups (Fig. 1). Interestingly, between the NGR and T2DM groups, serum Annexin A2 concentrations were not statistically increased in the T2DM group, instead of an increasing trend, suggesting that cardiomyopathy might be the main source in the elevation of serum Annexin A2 under DbCM condition, in contrast, T2DM itself might be a minor contribution in this situation.
3.3. The relationship between serum Annexin A2 and other parameters.
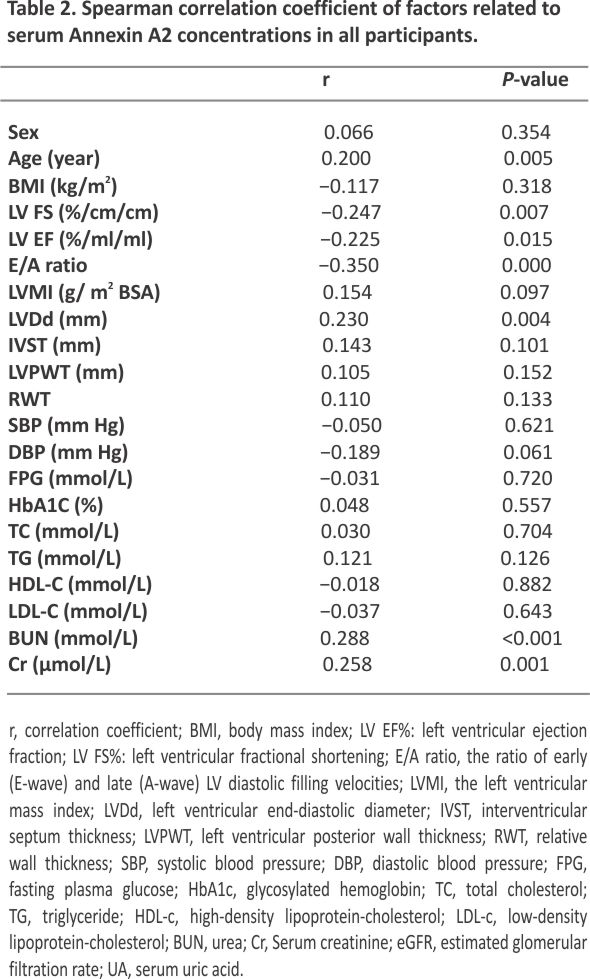
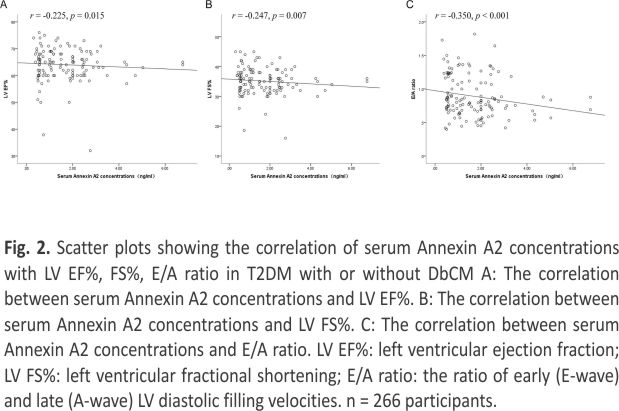
To further analyze the factors that potentially affected the serum Annexin A2 concentrations, we conducted Spearman’s correlation analysis. As shown in Table 2, serum Annexin A2 was positively associated with age, BUN, LVDd, and Cr, while negatively related with LV EF%, FS%, E/A ratio, and eGFR. All these correlations remained statistically significant after adjustment by age or in T2DM patients with or without DbCM (Fig. 2).
3.4. E/A ratio is the independent factor for Annexin A2 concentration in the multivariate logistic regression analysis
To further confirm the relationship between Annexin A2 concentration and DbCM and to find the independent factor affecting serum Annexin A2, multivariate logistic regression analysis was conducted and the Annexin A2 levels were divided into 3 groups by tertiles (tertile1 < 0.87; tertile2 0.87–1.72; tertile3 > 1.72 ng/mL). Both tertile1 and tertile3 of Annexin A2 concentration were significantly associated with DbCM (odds ratio, 2.59, 95% confidence interval, 1.27–5.30, odds ratio, 5.64, 95% confidence interval, 2.51–12.68, respectively). Moreover, the E/A ratio is the independent factor for Annexin A2 concentration (odds ratio, 0.17, 95% confidence interval, 0.03–0.95, respectively) when adjusted for age, LV EF%, BUN, and Cr (FS% and eGFR were not included in this analysis as collinearity). These results indicated that higher Annexin A2 circulating levels might predict a higher prevalence of DbCM, especially for poor LV diastolic function.
4. Discussion
As mentioned before, DbCM is defined as the presence of abnormal cardiac structure and functions but the absence of other cardiac risk factors, such as CAD, hypertension, and significant valvular disease16. In the myocardium, Annexin A2 is localized in intramyocardial capillaries, extracellular matrix, and endothelial cells of the coronary arteries8. Previous studies found it circulating levels were increased in hypertrophied or failing hearts 17, 18,19. Moreover, its circulating concentrations were also linked to microvascular complications of diabetes, including diabetic kidney disease and retinopathy 11. A current cross-sectional study revealed that serum Annexin A2 concentrations were increased in patients with DbCM when compared with T2DM without cardiac disease and normal glucose-tolerant subjects. It was negatively associated with LV EF, LV FS, E/A ratio, and eGFR, and was positively correlated with age, BUN, and Cr. These results suggested that serum Annexin A2 might be a biomarker for DbCM and cardiac systolic and diastolic functions.
In several animal and cellular studies, Annexin-A2 (encoded by gene ANXA2) has been identified as an endogenous inhibitor of proprotein convertase subtilisin/ kexin type-9 (PCSK9), which inhibits PCSK9-mediated degradation of LDL-R, maintains LDL-R levels at the cell surface, and promotes clearance of LDL-C 20,21. Fairoozy et al. 22 found that both rs11633032 and rs17191344 single nucleotide polymorphism (SNP) variants in Annexin A2 were associated with a higher risk of coronary atherosclerotic heart disease (CAD). Consistently, a recent cross-sectional study conducted by Lin et al. 10 in patients with CAD found that circulating Annexin A2 concentrations were significantly elevated in patients with CAD, especially in those with acute coronary syndrome, further confirming the deep association between circulating Annexin A2 levels and CAD. Thus, except for the failing heart, circulating Annexin-A2 concentrations were also related to lipid profiles and CAD. Notably, unlike the above findings, subjects recruited in our current study were screened by coronary CTA and excluded from the CAD. Moreover, we also failed to find the association between Annexin A2 and lipid profiles.
The early stage of DbCM is characterized by impaired myocardial diastolic function and increased atrial filling and enlargement, as well as elevated LV end-diastolic pressure 23. The following stage of DbCM is characterized by LV hypertrophy, cardiac remodelling, advancing cardiac diastolic dysfunction, and the consequent emergence of clinical indications of heart failure with normal ejection fraction 24. In the current study, although bivariate analysis suggested that serum Annexin A2 levels were related to both cardiac systolic and diastolic functions, the regressive analysis further revealed that only the E/A ratio was the independent factor of Annexin A2, in contrast to systolic indicators EF% and FS%, indicating that serum Annexin A2 concentrations might be used as an early predictive biomarker for DbCM. The underlying mechanisms regarding how Annexin A2 links to DbCM remain unclear. Nevertheless, based on current population findings, Annexin A2 might be involved in the pathological process of DbCM, and further investigations are necessary to figure out this possibility.
As for how Annexin A2 links with DbCM, previous evidence has implied several possible pathways and suggested that Annexin A2 regulated obesity-induced insulin resistance by interacting with the NF-κB signalling pathway 25 or galectin-3 26. In high-fat diet (HFD) mice, Annexin A2 knockdown elevated expressions of phosphorylated insulin receptor substrate 1 (IRS1) and peroxisome proliferator-activated receptor coactivator-1a, improved glucose tolerance and insulin sensitivity, and inhibited inflammatory response 25. Moreover, in Her-2 negative breast cancer cells, Annexin A2 interacted with galectin-3 at the cell surface, which bound directly to the insulin receptor, suppressed the down-stream signalling of IRS1 tyrosine phosphorylation and resulted in hyperglycemia 26, 27,28. Considering that hyperglycemia, insulin resistance, and inflammation were all well-recognized pathological factors for DbCM, Annexin A2 might contribute to DbCM and its progression through these mechanisms, while more research is needed to illustrate this in the future. In addition, the current study showed a positive relationship between serum Annexin A2 and renal dysfunction (increased blood BUN and Cr, and decreased eGFR), suggesting it might also be associated with diabetic kidney disease (DKD), another common complication of diabetes. However, it is well-recognized that heart failure is deeply linked with chronic kidney disease (CKD). Approximately 40% to 50% of heart failure patients have CKD 29 and vice versa, and up to 50% of CKD patients with either an ejection fraction preserved or reduced heart failure 30. Although our bivariate correlation analysis suggested that serum Annexin A2 was positively associated with both cardiac and renal dysfunctions, further regression analysis indicated cardiac function was the independent factor after adjusting renal function indexes. Thus, the current link between Annexin A2 and renal dysfunction may be second to DbCM, while more studies were necessary to further confirm this point.
As to the limitation of the current study, firstly, due to the cross-sectional design, the causal relationship between serum Annexin A2 and DbCM could not be concluded, and further basic and clinical experiments are needed to further elucidate the role of Annexin A2 in DbCN. Secondly, according to the previous investigation, age and renal functions could deeply affect serum Annexin A2 concentrations. Indeed, the current study found a significant association between serum Annexin A2 concentrations and renal functions including BUN, Cr, and eGFR. Nevertheless, after adjusting age and renal functions, higher Annexin A2 concentrations were still statistically related to DbCM.
In conclusion, serum Annexin A2 concentrations were higher in DbCM patients when compared with T2DM and NGR subjects. Circulating Annexin A2 was statistically, therefore our current study identified a circulating biomarker of Annexin A2 for cardiac diastolic function and early diagnosis of DbCM. Circulating Annexin A2 concentrations might be induced in DbCM patients and were negatively associated with cardiac systolic and diastolic functions, which suggested it might be a predictor of early diagnosis in DbCM and might be a potential therapeutic target for DbCM.
Ethics approval and consent to participate
All experimental protocols were approved by the Ethics Committee of Xinqiao Hospital, Third Military Medical University (Institutional Review Board–approved protocol number 2016-056-01). Written informed consent was collected for all subjects.
Consent for publication
All authors have read and approved the manuscript for publication.
Availability of data and materials
The clinical characteristic data of all human subjects used to support the findings of this study are included in the article.
Funding and Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82230025), the National Science Fund for Distinguished Young Scholars (No. 81925007) and Excellent Young Scholars (82122013), the National Natural Science Foundation of China (No. 82070836, No. 82270882, No. 82070881, No. 81970752, No. 82000769 and No. 82100910), and the “Talent Project” of Army Medical University (2019R012, 2019R047 and 2019XQYYYJ003-2).
Authors’ contributions
Q.H., statistical analysis; H.Q., XF.L., YR.W., X.X., and JR.Z.: analysis and interpretation of data; H.Q.: drafting of the manuscript; H.Q., HT.Z., and Y.Z.: critical revision of the manuscript for important intellectual content; H.Q., HT.Z., and Y.Z.: the guarantor of this work and, as such, had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
[1] S. Rubler, J. Dlugash, Y.Z. Yuceoglu, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis Am J Cardiol, 30 (6) (1972), pp. 595-602, 10.1016/00029149 (72)90595-4
[2] Y. Tan, Z. Zhang, C. Zheng, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence Nat Rev Cardiol, 17 (9) (2020), pp. 585-607, 10.1038/s41569-020-0339-2
[3] G. Borghetti, D. von Lewinski, D.M. Eaton, et al. Diabetic Cardio- myopathy: Current and Future Therapies Beyond Glycemic Control Front Physiol, 9 (2018), p. 1514, 10.3389/fphys.2018. 01514
[4] S. Boudina, E.D. Abel. Diabetic cardiomyopathy revisited Circulation, 115 (25) (2007), pp. 3213-3223, 10.1161/ circulationaha.106.679597
[5] A. Bharadwaj, E. Kempster, D.M. Waisman The Annexin A2/S10 0A10 Complex: The Mutualistic Symbiosis of Two Distinct Proteins Biomolecules, 11 (12) (2021), p. 1849, 10.3390/biom1112 1849
[6] I.A. Aliyu, K.H. Ling, N. Md Hashim, H.Y. Chee Annexin A2 extracellular translocation and virus interaction: A potential target for antivirus-drug discovery Rev Med Virol, 29 (3) (2019), p. e2038, 10.1002/ rmv.2038
[7] S. Mishra, V. Chander, P. Banerjee, et al. Interaction of annexin A6 with alpha actinin in cardiomyocytes BMC Cell Biol, 12 (7) (2011), 10. 1186/1471-2121-12-7
[8] E. Camors, V. Monceau, D. Charlemagne Annexins and Ca2+ handling in the heart Cardiovasc Res, 65 (4) (2005), pp. 793-802, 10.1016/j. cardiores.2004.11.010
[9] Y. Liu, H.K. Myrvang, L.V. Dekker Annexin A2 complexes with S100 proteins: structure, function and pharmacological manipulation Br J Pharmacol, 172 (7) (2015), pp. 1664-1676, 10.1111/bph.12978
[10]Z. Lin, D. Liu, Y. Xue, et al. [Serum annexin A2 level is significantly elevated in patients with coronary heart disease]. Nan fang yi ke da xue xue bao = J Southern Med Univ, 40 (3) (2020), pp. 382-387, 10.12122 /j.issn.1673-4254.20 20.03.16
[11].L. Lin, K. Hu. Annexin A2 and Kidney Diseases Front Cell Dev Biol, 10 (974381) (2022), 10.33 89/fcell. 2022. 974381
[12].K.G. Alberti, P.Z. Zimmet. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation Diabet Med: J Brit Diabet Assoc., 15 (7) (1998), pp. 539-553, 10. 1002/(SICI)1096-9136(199 807)15:7<539::AID-DIA668> 3.0.CO;2-S
[13].C.W. Yancy, M. Jessup, B. Bozkurt, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol, 62 (16) (2013), pp. e17 -e239, 10.1016/j.jacc.2013. 05.019
[14].H. Qu, M. Deng, H. Wang, et al. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance J Clin Lipidol, 9 (3) (2015), pp. 289-294,10.1016/j.jacl.2015. 03.006
[15]A.S. Levey, J.P. Bosch, J.B. Lewis, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group Ann Internal Med, 130 (6) (1999), pp. 461-470, 10.7326/0003-4819-130-6-199903160-00002
[16]G. Borghetti, D. von Lewinski, D.M. Eaton, et al. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control Front Physiol, 9 (2018), p. 1514, 10.3389/fphys. 2018.01514
[17]D. Benevolensky, Y. Belikova, R. Mohammadzadeh, et al. Expression and localization of the annexins II, V, and VI in myocardium from patients with end-stage heart failure Lab Invest, 80 (2) (2000), pp. 123-133, 10.1038/labinvest. 3780016
[18]P. Trouve, S. Legot, I. Belikova, et al. Localization and quantitation of cardiac annexins II, V, and VI in hypertensive guinea pigs Am J Physiol, 276 (4) (1999), pp. H1159-1166, 10.1152 /ajpheart. 1999.276.4.H1159
[19]G. Song, B. Campos, L.E. Wagoner, J.R. Dedman, R.A. Walsh Altered cardiac annexin mRNA and protein levels in the left ventricle of patients with end-stage heart failure J Mol Cell Cardiol, 30 (3) (1998), pp. 443-451, 10.1006/jmcc.1997. 0608
[20] K. Ly, Y.G. Saavedra, M. Canuel, et al. Annexin A2 reduces PCSK9 protein levels via a translational mechanism and interacts with the M1 and M2 domains of PCSK9 J Biol Chem, 289 (25) (2014), pp. 17732-17746, 10.1074/jbc.M113.54 1094
[21]G. Mayer, S. Poirier, N.G. Seidah. Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels J Biol Chem, 283 (46) (2008), pp. 31791-31801,10. 1074/jbc. M805971200
[22]R.H. Fairoozy, J. Cooper, J. White, et al. Identifying low density lipoprotein cholesterol associated variants in the Annexin A2 (ANXA2) gene Atherosclerosis, 261 (2017), pp. 60-68, 10.1016/j.athero- sclerosis.2017.04.010
[23] F. Westermeier, J.A. Riquelme, M. Pavez, et al. New Molecular Insights of Insulin in Diabetic Cardiomyopathy Front Physiol, 7 (125) (2016), 10.3389/fphys. 2016.00125
[24]G. Jia, V.G. DeMarco, J.R. Sowers Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy Nat Rev Endocrinol, 12 (3) (2016), pp. 144-153, 10.1038/nrendo.20 15. 216
[25]Y. Wang, Y.S. Cheng, X.Q. Yin, G. Yu, B.L. Jia Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the NF-kappaB signaling pathway Am J Physiol Cell Physiol, 316 (2) (2019), pp. C223-C234, 10.1152/ajpcell. 00242.2018
[26]P. Shetty, A. Bargale, B.R. Patil, et al. Cell surface interaction of annexin A2 and galectin-3 modulates epidermal growth factor receptor signaling in Her-2 negative breast cancer cells Mol Cell Biochem, 411 (1–2) (2016), pp. 221-233, 10.1007/s11010-015-2584-y
[27]P. Li, S. Liu, M. Lu, et al. Hematopoietic-Derived Galectin -3 Causes Cellular and Systemic Insulin Resistance Cell, 167 (4) (2016), pp. 973-984.e912, 10.1016/j.cell.2016.10.025
[28]H. Storgaard, X.M. Song, C.B. Jensen, et al. Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients [corrected] Diabetes, 50 (12) (2001), pp. 2770-2778, 10.23 37/diabetes.50.12.2770
[29]K. Damman, M.A. Valente, A.A. Voors, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis Eur Heart J, 35 (7) (2014), pp. 455-469, 10.1093/ eurheartj/eht386
[30]A. Tedeschi, P. Agostoni, B. Pezzuto, et al. Role of comorbidities in heart failure prognosis Part 2: Chronic kidney disease, elevated serum uric acid. Eur J Prev Cardiol, 27 (2_suppl) (2020), pp. 35-45, 10.1177/20474873 2095 7793.
Credits: Qingshan He, Jiaran Zhu, Guojun Yang, Xiufei Liu, Lu Li, Yuren Wang, Xin Xiong, Yi Zheng, Hongting Zheng, Hua Qu. Serum Annexin A2 concentrations are increased in patients with diabetic cardiomyopathy and are linked to cardiac dysfunctions, Diabetes Research and Clinical Practice, Volume 195, 2023, 110196, ISSN 0168-8227, https://doi.org/10.1016/j.diabres.2022.110196.
(https://www.sciencedirect.com/science/article/pii/S0168822722010105)