Warren Fong, Ting Hui Woon, Li-Ching Chew, Andrea Low, Annie Law, Yih Jia Poh, Siaw Ing Yeo, Ying Ying Leung, Margaret Ma, Amelia Santosa, Kok Ooi Kong, Chuanhui Xu, Gim Gee Teng, Anselm Mak, Sen Hee Tay, Tyng Yu Chuah, Nur Emillia Roslan, Stanley Angkodjojo, Kee Fong Phang, Melonie Sriranganathan, Teck Choon Tan, Peter Cheung & Manjari Lahiri
Abstract
Objective
To determine the prevalence and factors associated with flares post-Coronavirus disease 2019 (COVID-19) mRNA vaccination in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and spondyloarthritis (SpA).
Methods
A retrospective multi-centre study was conducted (January 2021 to February 2022). Data were collected during index visits, defined as the first post-vaccine visit in which the patient had a physician-defined flare, or if at least 3 months had elapsed since the first vaccine dose, whichever came first. Factors associated with flares were identified using mixed effects Cox regression and expressed as hazard ratio (HR) and 95% confidence interval (CI).
Results
A total of 2377 patients were included (1563 RA, 415 PsA, and 399 SpA). Among patients with RA, PsA, and SpA, 21.3%, 24.1%, and 21.8% experienced a flare respectively. Of those who experienced a flare, only 10.2%, 11.0%, and 14.9% were severe in patients with RA, PsA, and SpA respectively. Patients with low or moderate/high disease were more likely to flare compared to those in remission in patients with RA only (HR: 1.68, 95% CI 1.22– 2.31; HR: 2.28, 95% CI 1.50–3.48, respectively). Receiving the Moderna vaccine was associated with a higher HR of flare compared to the Pfizer vaccine in patients with PsA only (HR: 2.21, 95% CI 1.20–4.08). Patients who had two vaccine doses were found to be less likely to flare (HR: 0.08, 95% CI 0.06– 0.10). HRs of flares were not significantly different among RA, PsA, and SpA.
Conclusion
About one-fifth of patients experienced a disease flare post-COVID-19 mRNA vaccination, but most flares were non-severe. Patients with active disease prior to vaccination should be monitored closely for disease flares, especially in patients with RA.
Introduction
As compared to the general population, patients with autoimmune inflammatory rheumatic diseases (AIIRD) are more vulnerable to contracting Coronavirus disease 2019 (COVID-19) 1 and are at risk of poorer outcomes 2,3,4. Being vaccinated is effective in reducing the risk of COVID-19 and in improving disease-related outcomes such as hospitalisation and mortality in these patients 5,6,7. The Pfizer-BioNTech and Moderna vaccines were the first two vaccines approved for use in Singapore and rely on mRNA technology. However, these vaccines bring about a theoretical risk of inducing AIIRD flares—a common barrier against vaccination 8,9,10—due to the production of neutralising antibodies and molecular mimicry 11,12,13. In addition, the lipid nanoparticles used to encapsulate the mRNA for better vaccine delivery have self-adjuvant properties that might stimulate inflammatory responses 14,15.
Several observational studies and registries were set up to obtain more data about the safety of COVID-19 vaccinations in patients with AIIRD, which reflected about 2–11% of disease flares 16,17,18,19,20. However, there were limited studies targeted at patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and spondyloarthritis (SpA) and factors associated with disease flares in these patients. We aimed to determine the prevalence and factors for flares in these patients after receiving the COVID-19 mRNA vaccination.
Patients and methods
Study design and population
This is a sub-study of a multi-center retrospective cohort study (COronavirus National Vaccine Registry for ImmuNe diseases Singapore) conducted across eight hospitals in Singapore 21, reported in line with the Strengthening of Reporting in Observational studies in Epidemiology (STROBE) guidelines 22 (Additional file 1). Patients clinically diagnosed with RA, PsA, or SpA by a rheumatologist, aged 12 years and above and who received at least one dose of an mRNA vaccine (Pfizer-BioNTech or Moderna) were included. National Healthcare Group Domain Specific Review Board approved this study (Reference number: 2021/00433). A waiver of informed consent was approved for the study.
Data collection
Data were collected from January 2021 through February 2022 through medical records review and entered into an anonymised, secure, online portal. A standardized questionnaire was used for data collection and data fields included the patient’s age, sex, race, type of COVID-19 vaccine received, dates of first and second dose of the vaccine, date of post-vaccination index visit, primary diagnosis, physician-defined disease activity prior to vaccination (remission, low, moderate or high disease activity), immunomodulatory/ immunosuppressive treatment received at the time of vaccination, whether treatment was interrupted for purposes of the vaccine and whether there was a physician-defined flare at the index visit. We followed up with participants from their first vaccine dose till index visit, which was defined as the first post-vaccine visit when the patient had flared, or if at least 3 months had elapsed since the first dose of the vaccine, whichever came first. The primary outcome of interest was a physician-defined flare that occurred within 3 months after the first vaccine dose.
If there was a flare, additional data fields included the date of the flare, the severity of the flare, and whether hospitalisation was required due to the flare. For patients with SpA and PsA, data on extra-musculoskeletal flares were collected. A mild, self-limiting flare was defined as any flare that did not necessitate treatment escalation or early consultation with the rheumatologist, and resolved before the index visit. Moderate flares included any flares that required an early rheumatologist consult and/or increase in treatment (but not exceeding a daily prednisolone dose of 20 mg, requiring intramuscular or intra-articular glucocorticoid injection, initiation of new biological disease-modifying anti-rheumatic agents (bDMARDs) or the initiation of cytotoxic drugs, such as cyclophosphamide). A severe flare was defined as any flare that required hospitalization, a daily prednisolone dose of more than 20 mg, intramuscular or intra-articular glucocorticoid injection, initiation of bDMARDs, or initiation of cytotoxic drugs.
Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR) and compared using the Kruskal–Wallis test. Categorical variables were expressed as numbers (percentages), and compared using the Chi-square test or Fisher’s exact test, as appropriate. Factors associated with flares were identified using multivariable mixed effects Cox regression, while accounting for the clustering effect by Singapore hospital clusters 23, and expressed as hazard ratio (HR) and 95% confidence interval (CI). Potential factors included diagnosis, age, gender, race, disease activity, type of vaccine, and number of vaccine doses 16,18,24. Patients who fulfilled the primary outcome (flared within 3 months from the first vaccine dose) and who did not flare were included in the mixed effects Cox regression. A sensitivity analysis that included all patients in the regression model was also performed. All statistical analyses were conducted using Stata SE 15 (StataCorp LLC, Texas, USA) and a p-value of < 0.05 was considered to be statistically significant. The design of the online data entry portal ensured that there was no missing data.
Results
Patient characteristics
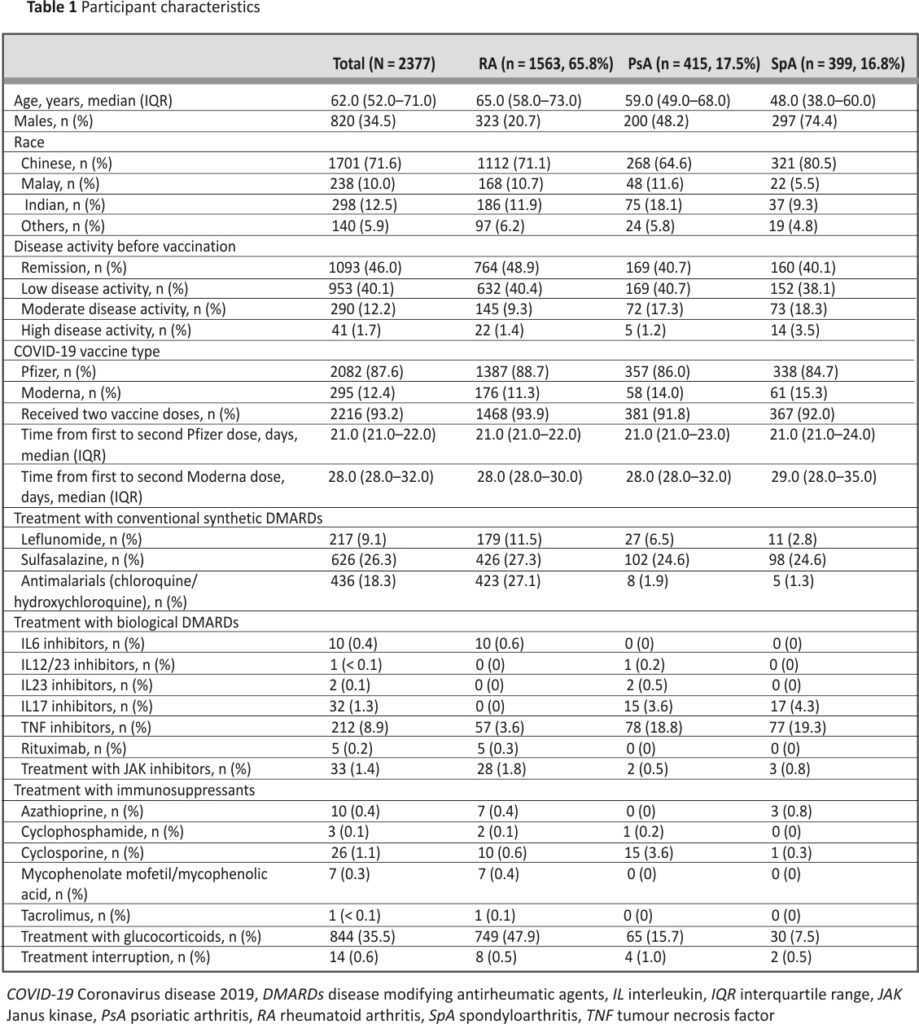
Patient characteristics are shown in Table 1. A total of 2377 patients were included from January 2021 to February 2022. The median (IQR) age was 62.0 (52.0–71.0) years and 820 (34.5%) were male subjects. Out of all patients, 1701 (71.6%) were Chinese, 1093 (46.0%) were in disease remission before vaccination, 2082 (87.6%) received the Pfizer vaccine and 2216 (93.2%) received two vaccine doses in total. Only 14 (0.6%) had interruption of treatment due to the vaccine.
In terms of disease-modifying anti-rheumatic drugs (DMARDs), patients with RA and PsA were mostly on methotrexate (58.9% and 54.9% respectively), whilst patients with SpA were mostly on sulfasalazine (24.6%). In terms of biological DMARDs, the majority of patients were on TNF inhibitors (RA: 3.6%, PsA: 18.8%, SpA: 19.3%).
Information regarding flares
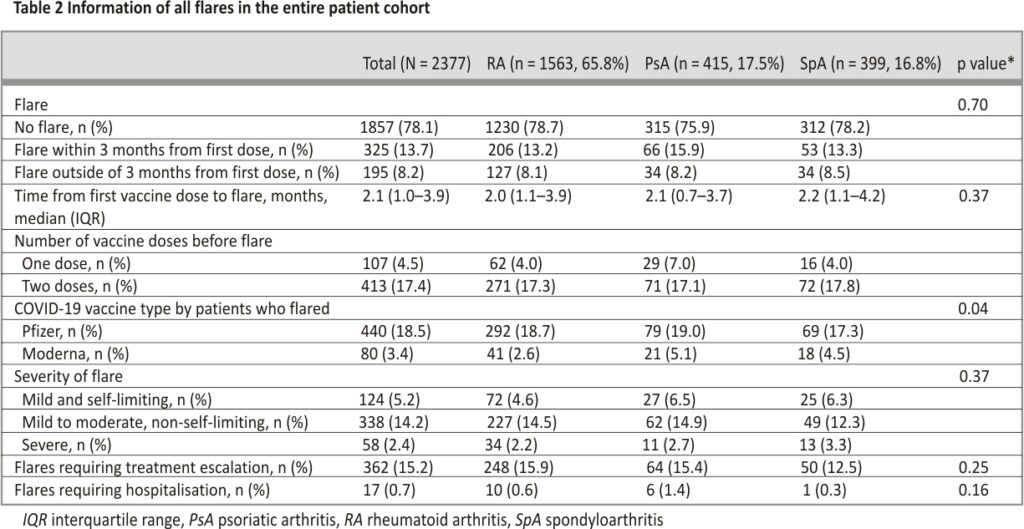
Information regarding flares (no flare, flared within 3 months from first dose and outside of 3 months from first dose) is shown in Tables 2 and 3. The median (IQR) follow-up duration from the first vaccine dose to index visit was 4.4 (3.5– 5.5) months, of which 1857 (78.1%) did not experience a flare, and the prevalence of flare was not significantly different between the three groups. Five-hundred and twenty (21.9%) flares were observed over 10,362.8 patient-months of follow-up (incidence rate of 5.0/ 100 patient-months), of which 325 (13.7%) experienced a flare within 3 months from the first vaccine dose and 195 (8.2%) flared outside of the 3 months’ time frame. The median (IQR) time to flare for all patients was 2.1 (1.0–3.9) months and was not significantly different between the three groups.
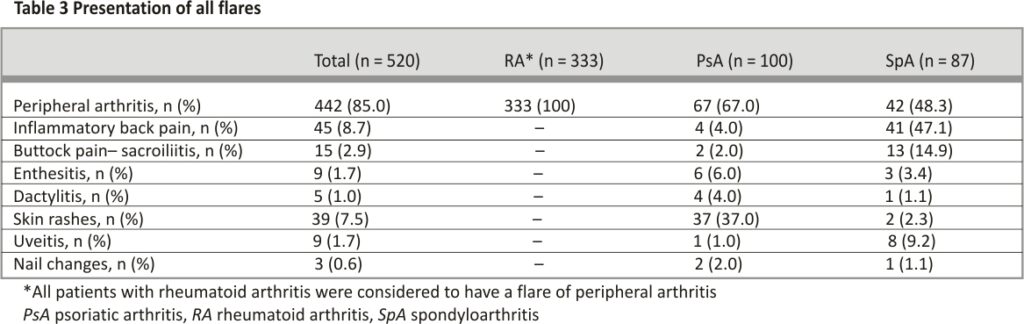
Amongst patients who experienced a disease flare, 107 (20.6%) had a flare after one vaccine dose while 413 (79.4%) flared after two doses. Four-hundred and forty (84.6%) had the Pfizer vaccine and 80 (15.4%) had Moderna. Only a minority had a severe flare (RA: 10.2%, PsA: 11.0%, SpA 14.9%). Of the patients that had a disease flare, the majority required treatment escalation (RA: 74.5%, PsA: 64.0%, SpA 57.5%) while few required hospitalization (RA: 3.0%, PsA: 6.0%, SpA 1.1%). Among patients with RA who flared, all had a flare of peripheral arthritis. Patients with PsA mainly had a flare of peripheral arthritis (67.0%) and psoriasis (37.0%), whilst patients with SpA mainly had a flare of peripheral arthritis (48.3%) and inflammatory back pain (47.1%).
Factors associated with flares after vaccination
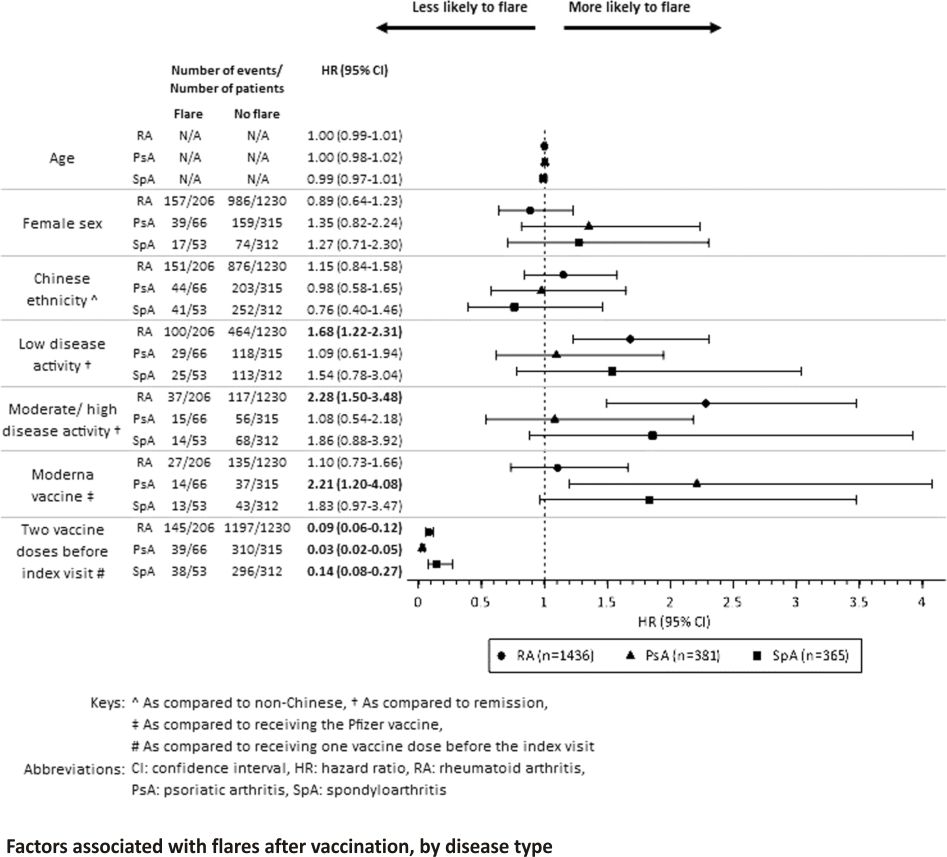
Factors associated with flares after vaccination are shown in Table 4 and Fig. 1. Higher disease activities in patients with RA before vaccination were associated with experiencing a flare, whereas patients with low or moderate/high disease activities were more likely to flare compared to those in remission (HR: 1.68, 95% CI 1.22–2.31; HR: 2.28, 95% CI 1.50–3.48, respectively). Disease activity was not correlated with the risk of flare in patients with PsA and SpA.
Patients who received the Moderna vaccine were more likely to flare compared to those who received the Pfizer vaccine in patients with PsA (HR: 2.21, 95% CI 1.20–4.08), but not in patients with RA and SpA. Patients who had two vaccine doses were found to be less likely to flare (HR: 0.08, 95% CI 0.06–0.10), and this was not significantly different across the three groups. Results from the sensitivity analysis were largely similar (Additional file 2). Compared to RA, the hazard ratio of disease flare was not significantly different in patients with PsA or SpA.
Multivariable mixed effects Cox regression analyses were conducted to identify factors associated with having a flare while accounting for the clustering effect by Singapore hospital clusters. Patients who fulfilled the primary outcome (flared within 3 months from the first vaccine dose) and who did not flare were included in the analyses.
Discussion
The prevalence of a flare was seen in 21.3%, 24.1%, and 21.8% of patients, and severe flares occurred in 10.2%, 11.0%, and 14.9% of the patients with RA, PsA, and SpA respectively. This study reported a higher rate of flare compared to previous studies, where rates ranged from 2 to 11%, possibly due to differences in study methodologies. Previous studies that required a change in medication or outpatient consultation to be considered as a flare were voluntary reports by patients or physicians, or had a shorter follow-up period from vaccination to index visit (7 days–3 months) 16,17,18,19,20. In our study, however, flares were physician-defined and included those that were mild and self-limiting in nature and did not require up-titration of treatment.
Local guidelines for patients with rheumatic diseases advised that interruption of immunomodulatory drugs for the purposes of COVID-19 vaccination is not necessary, with the exception of rituximab 25. About 0.6% of patients in this study had treatment interruption during vaccination, which was higher than the percentage of patients receiving rituximab (0.2%). A possible reason might be that patients stopped their medications prior to vaccination without consulting their physicians 26, 27.
This study identified patients with higher disease activities prior to vaccination as being more likely to flare, similar to previous studies which showed that having a flare 6–12 months prior to receiving the vaccine was associated with experiencing a flare thereafter, and that stable disease activity was a negative predictor of flares 20, 28,29,30. Amongst patients who flared, only 11.2% and 3.3% of patients experienced severe flares and flares requiring hospitalisation, respectively, and this supports recommendations from both local and international guidelines that advocate for the safety of vaccination in patients with active disease, except in patients afflicted with life-threatening illnesses 25,31,32. Patients who took the Moderna vaccine were also found to be more likely to flare, possibly due to the higher mRNA content in the Moderna vaccine 33,34, although prior studies did not prove disease flares to be associated with the type of mRNA vaccine 19,35. Receiving two doses of the vaccine was also associated with a lower risk of flare, possibly because patients who experienced adverse events after the first vaccination would be less likely to proceed with the second dose. However, 17.4% of all patients flared after the second dose which might highlight the need for close monitoring of patients even if they did not experience any adverse events after the first dose. Age was not found to be associated with experiencing a flare, in contrast with another study which showed that being elderly was a risk factor for a flare of underlying AIIRDs 28. In this study, we did not observe significant differences in the hazard ratios for flares between patients with RA, PsA, or SpA, in contrast to the Global Rheumatology Alliance registry, which found that patients with PsA were more likely to flare as compared to RA 16.
Strengths of this study include the large sample size and the inclusion of patients of a large age range, which increases the generalisability of results. Patient demographics were also similar to previous reports 36,37. We required the index visit to be at least 3 months after the first vaccine dose for cases with no flares to ensure a sufficient observation period. Flares not requiring a change in treatment were also included to obtain a more comprehensive spectrum of data as some patients might not seek a change in medication for their flares 38. The study was also conducted using a detailed chart review rather than just using administrative data collected, and flares were physician-defined and documented in the clinical notes.
Given the retrospective nature of this study, results are prone to information bias and there are also several limitations that restrict the interpretation of results. There was no control group (non-vaccinated patients with AIIRD). Given the rigorous vaccination efforts conducted locally in line with national policies, the number of unvaccinated individuals in Singapore was low (only 7% of the population unvaccinated) 39. Also, no causal relationship between taking the vaccine and experiencing a flare can be deduced from this study. As this study was initiated after the COVID-19 pandemic, data on flares prior to the pandemic and vaccination were unavailable, which might restrict the interpretation of findings from this study. Although previous literature had reported on the background rates of flares before vaccine availability 38,40,41, definitions of flare were heterogeneous and no definitive comparisons could be made. In addition, myalgias and arthralgias are well-described side effects of the mRNA vaccines, which could be mistaken to be a disease flare. Adverse events which could overlap with manifestations of rheumatic diseases were also not collected. However, we had aimed to reduce any misclassification by using a physician-defined flare as the outcome of interest as well as a standardized questionnaire for data collection. Prior to study initiation, a consensus meeting was held and practice cases were also used to harmonise definitions of pre-vaccine disease activity and adjudication of flare. Although we collected details of the dates of the first and second vaccinations, data collection was truncated after the first flare. For patients who flared after the first dose, we were unable to determine the prevalence and severity of flares should they proceed with the second dose, which might be important information to patients. Data for other potential confounders such as comorbidities were also not collected in this study 42.
Conclusions
This study showed that about one in five patients with RA, PsA, and SpA experienced a disease flare post-COVID-19 mRNA vaccination, but most flares were non-severe. Patients with active disease prior to vaccination should be more closely monitored for disease flares post-vaccination, especially for patients with RA.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank all staff involved for their contribution.
Funding
This study did not receive any funding.
Author information
Author notes
Warren Fong and Ting Hui Woon Co-first authors.
Authors and Affiliations
Department of Rheumatology and Immunology, Singapore General Hospital, Academia, Level 4, 20 College Road, Singapore, 169856, Singapore
Warren Fong, Ting Hui Woon, Li-Ching Chew, Andrea Low, Annie Law, Yih Jia Poh, Siaw Ing Yeo & Ying Ying Leung
Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Warren Fong, Li-Ching Chew, Andrea Low, Margaret Ma, Amelia Santosa, Gim Gee Teng, Anselm Mak, Sen Hee Tay, Kee Fong Phang, Peter Cheung & Manjari Lahiri
Rheumatology, Duke-NUS Medical School, Singapore, Singapore
Warren Fong, Li-Ching Chew, Andrea Low, Annie Law & Ying Ying Leung
Division of Rheumatology, Department of Medicine, National University Hospital, Singapore, Singapore
Margaret Ma, Amelia Santosa, Gim Gee Teng, Anselm Mak, Sen Hee Tay, Peter Cheung & Manjari Lahiri
Rheumatology, Tan Tock Seng Hospital, Singapore, Singapore
Kok Ooi Kong & Chuanhui Xu
Rheumatology, Sengkang General Hospital, Singapore, Singapore
Tyng Yu Chuah, Nur Emillia Roslan & Stanley Angkodjojo
Department of Medicine, Alexandra Hospital, Singapore, Singapore
Kee Fong Phang
Rheumatology, Changi General Hospital, Singapore, Singapore
Melonie Sriranganathan
Rheumatology, Khoo Teck Puat Hospital, Singapore, Singapore
Teck Choon Tan
Contributions
All authors except THW conceptualized the study design. All authors acquired the data. WF and THW wrote the initial manuscript draft. All authors critically revised the article for important intellectual content and approved of the final manuscript.
Corresponding author
Correspondence to Warren Fong.
Abbreviations
AIIRD: Autoimmune inflammatory rheumatic diseases
CI: Confidence interval
COVID-19: Coronavirus disease 2019
DMARDs: Biological disease-modifying anti-rheumatic drugs
HR: Hazard ratio
IQR: Interquartile range
PsA: Psoriatic arthritis
RA: Rheumatoid arthritis
SpA: Spondyloarthritis
SD: Standard deviation
STROBE: Strengthening of Reporting in Observational Studies in Epidemiology
TNF: Tumour necrosis factor
References
1. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021; 80:384–91. https://doi.org/10.11 36/annrheumdis-2020-2189 46.
2. Conway R, Grimshaw AA, Konig MF, et al. SARS–CoV-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol. 2022;74:766–75. https: //doi.org/10.1002/art.4 2030.
3. Xu C, Yi Z, Cai R, Chen R, Thong BYH, Mu R. Clinical outcomes of COVID-19 in patients with rheumatic diseases: A systematic review and meta-analysis of global data. Autoimmun Rev. 2021; 20:102778. https://doi.org/ 10.1016/j.autrev.2021.102778.
4. Qi G, Wang H, Guo Y, et al. Clinical outcomes of COVID-19 patients with rheumatic diseases: a retrospective cohort study and synthesis analysis in Wuhan. China Clin Rheumatol. 2022; 41:1899–910. https://doi.org/ 10.1007/s10067-022-06086-2.
5. Bieber A, Sagy I, Novack L, et al. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: a national cohort study. Ann Rheum Dis. 2022; 81:1028 –35. https://doi.org/10.1136 /annrheumdis-2021-221824.
6. Papagoras C, Fragoulis GE, Zioga N, et al. Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Ann Rheum Dis. 2022;81: 1013–6. https://doi.org/10.1136/ annrheumdis-2021-221539.
7. Widdifield J, Kwong JC, Chen S, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. 2022; 4:E430–40. https://doi.org/10.1016/S2665 -9913(22)00 096-0.
8. Roberta P, Greta P, Serena C, et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. 2021;80:953 –4. https://doi.org/10.1136 /annrheumdis-2021-220059.
9. Felten R, Dubois M, Ugarte-Gil MF, et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021; 3:e243. https://doi.org/10. 1016/S2665-9913(21)00 039-4.
10. Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021; 7:e001814. https://doi.org/10. 1136/RMDOPEN-2021 -001814.
11. Luchetti Gentiloni MM, Paci V, Marconi V, et al. SARS-COV-2 infection, vaccination, and immune-mediated diseases: results of a single-center retrospective study. Front Immunol.2022;13:859550. https://doi.org/10.3389/fimmu.2022.859550.
12. Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3:e469–70. https://doi.org /10.1016/S2665-9913(21)00 10 -9.
13. Chen Y, Xu Z, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immu- nology.2021;165:386–401. https: //doi.org/10.1111/imm.1344 3.
14. Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586. https://doi.org/10.1016/j.ijpharm.2021. 120586.
15. Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021; 24:103479. https://doi. org/10.1016/j.isci.2021.103479.
16. Rider LG, Parks CG, Wilkerson J, et al. Baseline factors associated with self-reported disease flares following COVID-19 vaccination among adults with systemic rheumatic disease: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. Rheu- matol. 2022;61:143–50. https://doi. org/10.10903/ RHEUMATO LOGY/KEAC249.
17. Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS- CoV- 2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2021;81:695–709. https://doi.org/ 10.1136/annrheumdis-2021-22 1490.
18. Fragoulis GE, Bournia VK, Mavrea E, et al. COVID-19 vaccine safety and nocebo-prone associated hesitancy in patients with systemic rheumatic diseases: a cross-sectional study. Rheumatol Int. 2022;42:31–9. https://doi. org/10.1007/S00296-021-05 039-3.
19. Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021;81:564–8. https://doi.org/10.1136/annrheumdis-2021-221571.
20. Connolly CM, Ruddy JA, Boyarsky BJ, et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS–CoV-2 messenger RNA vaccination. Arthritis Rheumatol. 2022;74:28–32. https://doi. org/10.1002/ART.41924.
21. Ma M, Santosa A, Kong KO, et al. Post-mRNA vaccine flares in autoimmune inflammatory rheumatic diseases: results from the COronavirus National Vaccine registry for ImmuNe diseases SINGapore (CONVIN-SING). Ann Rheum Dis. 2022; 81:333–4. https://doi.org/10.1136/ANN RHEUMDIS-2022-EULAR.1787.
22. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, – broucke JP. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007; 4:1623–7. https://doi.org/10. 1371/JOURNAL.PMED.0040296.
23. MOH. Reorganisation of the healthcare system into three integrated clusters to better meet future healthcare needs. Published 2017. Accessed August 15, 2022. https : //www.moh.gov.sg/news-highlights/details/reorganisation-of-healthcare-system-into-three-integrated-clusters-to-better-meet-future-health care-needs.
24. Xie Y, Liu Y, Liu Y. The flare of rheumatic disease after SARS-CoV-2 vaccination: a review. Front Immunol.2022;13:919 979. https://doi.org/10. 3389/FIMMU.2022.919979.
25. Santosa A, Xu C, Arkachaisri T, et al. Recommendations for COVID-19 vaccination in people with rheumatic disease: developed by the Singapore Chapter of Rheumatologists. Int J Rheum Dis. 2021; 24: 746–57. https://doi.org/10.1111/1756-185X.14107.
26. Zateri C, Birtane M, Aktaş İ, et al. Attitudes of patients with spondylarthritis or rheumatoid arthritis regarding biological treatment during COVID-19 pandemic: a multi-center, phone-based, cross-sectional study. Arch Rheumatol. 2021; 36:473. https: //doi.org/10.46497/ARCHRHEU MATOL.2021.8364.
27. Park JK, Lee EB, Shin K, et al. COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean College of Rheumatology. J Korean Med Sci. 2021;36:e95. https://doi.org/ 10.3346/JKMS.2021.36.E95.
28. Fan Y, Geng Y, Wang Y, et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real-world survey on inactivated COVID-19 vaccines. Ann Rheum Dis. 2022; 81:443 – 5. https://doi.org/10.1136/ annrheumdis-2021-221736.
29. Felten R, Kawka L, Dubois M, et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021;3:e613–5. https://doi.org/ 10.1016/S2665-9913(21)00221-6.
30. Rotondo C, Cantatore FP, Fornaro M, et al. Preliminary data on post-market safety profiles of COVID-19 vaccines in rheumatic diseases: assessments on various vaccines in use, different rheumatic disease subtypes, and immunosuppressive therapies: a two-center study. Vaccines. 2021;9:730. https://doi.org /10. 3390/VACCINES9070730.
31. Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis.2020;79:39–52. https://doi.org/10.1136/ annrheumdis-2019-215882.
32. Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73: e60–75. https://doi.org/10. 1002/ART.41928/ABSTRACT.
33. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT 162b2 and mRNA-1273. JAMA. 2021;326: 1533–5. https://doi. org/10.1001/JAMA.2021.1512 5.
34. Yau K, Chan CT, Abe KT, et al. Differences in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. Can Med Assoc J. 2022;194: E297–305. https://doi.org/10. 1503/cmaj.211881.
35. Pinte L, Negoi F, Ionescu GD, et al. The COVID-19 vaccine does not increase the risk of disease flare-ups among patients with autoimmune and immune-mediated diseases. J Pers Med. 2021;11:1283. https://doi.org /10.3390/JPM11121283/S1.
36. Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011. https:// doi.org/ 10.1002/art.30155.
37. Gladman DD, Ritchlin C. Clinical manifestations and diagnosis of psoriatic arthritis. UpToDate. Published 2022. Accessed November 21, 2022. https://www. uptodate.com/contents/clinical-manifestations-and-diagnosis-of-psoriatic-arthritis #H2
38. Bykerk VP, Shadick N, Frits M, et al. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol. 2014;41:227 –34. https://doi.org/10.3899/JRHE UM.121521.
39. MOH. MOH | Vaccination Statistics. Published 2022. Accessed June 4, 2022. https://www.moh.gov.sg /covid-19/vaccination/statistics.
40. Lubrano E, Perrotta FM, Manara M, et al. Predictors of loss of remission and disease flares in patients with axial spondylo- arthritis receiving antitumor necrosis factor treatment: a retrospective study. J Rheumatol. 2016;43:1541–6. https://doi. org/10.3899/jrheum.160363.
41. Bechman K, Tweehuysen L, Garrood T, et al. Flares in rheumatoid arthritis patients with low disease activity are hard to predict and strongly associated with worse clinical outcomes. J Rheumatol. 2018; 45:1515–21. https://doi.org/ 10.3899/JRHEUM.171375.
42. Chowdhury SD, Oommen AM. Epidemiology of COVID-19. J Dig Endosc. 2020;11:3–7. https://doi. org/10.1055/S-0040-1712187.
CREDIT: Fong, W., Woon, T.H., Chew, LC. et al. Prevalence and factors associated with flares following COVID-19 mRNA vaccination in patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis: a national cohort study. Adv Rheumatol 63, 38 (2023). https://doi.org/10. 1186/s42358-023-00316-0