Divaka Perera, Holly P. Morgan, Matthew Ryan, Matthew Dodd, Tim Clayton, Peter D. O’Kane, John P. Greenwood, Simon J. Walsh, Roshan Weerackody, Adam McDiarmid, George Amin-Youssef, Julian Strange, Bhavik Modi, Timothy Lockie, Kai Hogrefe, Fozia Z. Ahmed, Miles Behan, Nicholas Jenkins, Eltigani Abdelaal, Michelle Anderson, Stuart Watkins, Richard Evans, Christopher A. Rinaldi, and Mark C. Petrie, and for the REVIVED-BCIS2 Investigators
Correspondence to: Divaka Perera, MD, Cardiovascular Division, Kings College London, Rayne Institute, St Thomas’ Hospital, London SE1 7EH, United Kingdom. E-mail Address: divaka.perera@kcl.ac.uk
Abstract
BACKGROUND:
Ventricular arrhythmia is an important cause of mortality in patients with ischemic left ventricular dysfunction. Revascularization with coronary artery bypass graft or percutaneous coronary intervention is often recommended for these patients before implantation of a cardiac defibrillator because it is assumed that this may reduce the incidence of fatal and potentially fatal ventricular arrhythmias, although this premise has not been evaluated in a randomized trial to date.
METHODS:
Patients with severe left ventricular dysfunction, extensive coronary disease, and viable myocardium were randomly assigned to receive either percutaneous coronary intervention (PCI) plus optimal medical and device therapy (OMT) or OMT alone. The composite primary outcome was all-cause death or aborted sudden death (defined as an appropriate implantable cardioverter defibrillator therapy or a resuscitated cardiac arrest) at a minimum of 24 months, analyzed as a time to the first event on an intention-to-treat basis. Secondary outcomes included cardiovascular death or aborted sudden death, appropriate implantable cardioverter defibrillator (ICD) therapy or sustained ventricular arrhythmia, and a number of appropriate ICD therapies.
RESULTS:
Between August 28, 2013, and March 19, 2020, 700 patients were enrolled across 40 centers in the United Kingdom. A total of 347 patients were assigned to the PCI+OMT group and 353 to the OMT-alone group. The mean age of participants was 69 years; 88% were male; 56% had hypertension; 41% had diabetes; and 53% had a clinical history of myocardial infarction. The median left ventricular ejection fraction was 28%; 53.1% had an implantable defibrillator inserted before randomization or during follow-up. All-cause death or aborted sudden death occurred in 144 patients (41.6%) in the PCI group and 142 patients (40.2%) in the OMT group (hazard ratio, 1.03 [95% CI, 0.82–1.30]; P=0.80). There was no between-group difference in the occurrence of any of the secondary outcomes.
CONCLUSIONS:
PCI was not associated with a reduction in all-cause mortality or aborted sudden death. In patients with ischemic cardiomyopathy, PCI is not beneficial solely for the purpose of reducing potentially fatal ventricular arrhythmias.
REGISTRATION:
URL: https://www.clinicaltrials. gov; Unique identifier: NCT01920048.
Key Words: arrhythmias, cardiac ◼ cardiomyopathies ◼ death, sudden, cardiac ◼ defibrillators, implantable ◼ heart failure ◼ myocardial revascularization ◼ percutaneous coronary intervention
Clinical Perspective
What Is New?
• The rate of death or aborted sudden death remains high in ischemic left ventricular dysfunction, despite the use of guideline-directed medical therapy.
• Percutaneous coronary intervention was not associated with lower all-cause mortality, aborted sudden death, or sustained ventricular arrhythmias in patients with ischemic left ventricular dysfunction compared with optimal medical therapy alone.
What Are the Clinical Implications?
• Patients with ischemic left ventricular dysfunction should not undergo percutaneous coronary intervention with the sole aim of preventing the occurrence of ventricular arrhythmias.
• In stable patients who are treated with guideline-directed medical therapy and meet the criteria for implantable cardioverter defibrillator implantation, there is no evidence to support delaying implantation merely to assess the effect of percutaneous coronary intervention because the latter was not found to improve left ventricular function.
Ischemic left ventricular systolic dysfunction is associated with significant morbidity and mortality. The leading cause of death in this population is sudden cardiac death, resulting largely from ventricular arrhythmias.1,2Implantable cardioverter-defibrillators (ICDs) are widely used to mitigate the risk of sudden death in this population with primary prevention ICDs indicated in patients with a left ventricular ejection fraction <35%.3
The generation of ventricular arrhythmias in this population is believed to relate to both myocardial scarring and inducible ischemia.1,4 Treating the latter with coronary revascularization (with coronary artery bypass graft [CABG] or percutaneous coronary intervention [PCI]) is assumed to lead to improvement in left ventricular ejection fraction, which underlies contemporary guidelines that recommend deferral of ICD implantation for up to 90 days after revascularization in a primary prevention setting.5,6 Despite widespread clinical adoption, no high-quality data exist to support this approach. The recently published REVIVED-BCIS2 trial (Revasculari- sation for Ischaemic Ventricular Dysfunction–British Cardiovascular Intervention Society 2 Trial) found that revascularization with PCI did not reduce the incidence of all-cause death or hospitalization for heart failure in patients with ischemic left ventricular dysfunction.7 Among the subgroup who had an ICD or cardiac resynchronization therapy (CRT) defibrillator (CRT-D) implanted before randomization or within the first 90 days, those assigned to have PCI had fewer appropriate ICD therapies than those assigned to optimal medical therapy (OMT) alone, but this difference failed to reach statistical significance at 2 years. We now report the incidence of appropriate ICD therapies, resuscitated cardiac arrest, sustained ventricular arrhythmias, and death over the entire duration of follow-up in the PCI group compared with the group assigned to OMT alone.
Nonstandard Abbreviations and Acronyms
CABG – coronary artery bypass graft
CABG PATCH – Coronary Artery Bypass Graft Patch
CRT – cardiac resynchronization therapy
CRT-D cardiac resynchronization therapy defibrillator
DANISH-Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality
DAPA HF Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial
ICD implantable cardioverter-defibrillator
ISCHEMIA International Study of Comparative Health Effectiveness With Medical and Invasive Approaches
MADIT II Multicenter Automatic Defibrillator Implantation 2 Trial
MUSTT Multicenter Unsustained Tachycardia Trial
OMT optimal medical therapy
PCI percutaneous coronary intervention
PROTECT-II Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon Pump [IABP] in Patients Undergoing Non-Emergent High-Risk PCI
REVIVED-BCIS2 – Revascularisation for Ischaemic Ventricular Dysfunction–British Cardiovascular Intervention Society 2 Trial
STICH Surgical Treatment for Ischemic Heart Failure
METHODS
Study Design
The trial design and primary outcome results have been reported previously.7,8 In brief, REVIVED-BCIS2 was a prospective, randomized, multicenter open-label trial in which patients with ischemic left ventricular systolic dysfunction were randomized to receive either PCI plus OMT or OMT alone. Participants were recruited from 40 hospitals in the United Kingdom. The trial protocol was registered before the enrollment of the first patient (URL: https://www.clinicaltrials.gov. Unique identifier: NCT01920048; URL: https://www.isrctn.com. Unique identifier: ISRCTN 45979711) and was approved by the UK Health Research Authority. All patients provided written informed consent.
All participants enrolled in the REVIVED-BCIS2 trial were included in the present analyses. Patients were eligible for inclusion in the trial if they had ischemic left ventricular dysfunction, defined by the presence of severe left ventricular systolic dysfunction (left ventricular ejection fraction ≤35%), extensive coronary disease (British Cardiovascular Intervention Society Jeopardy Score ≥6), and evidence of viability in at least 4 dysfunctional myocardial segments that were amenable to revascularization with PCI. Those with myocardial infarction in the 4 weeks before randomization, sustained ventricular arrhythmia (ventricular tachycardia, ventricular fibrillation, or appropriate ICD discharges) in the 72 hours before randomization, or acutely decompensated heart failure were excluded.
The statistical analysis plan for this arrhythmia sub-study was finalized before the unblinding of trial group data from the arrhythmia case report form and is included in the Supplemental Material. The data that support the findings of this study and the analytic methods will be made available 1 year from the completion of the trial on reasonable request to the corresponding author.
Randomization and Data Collection
Participants were randomized in a 1:1 manner to either PCI plus OMT (PCI group) or OMT alone (OMT group). Randomization was stratified by center using randomly permuted blocks of varying size. Those randomized to the PCI group had revascularization attempted on all proximally diseased vessels subtending viable myocardium. All patients were initiated on OMT before enrollment, with subsequent up-titration of therapy supervised by a heart failure specialist at each recruiting site. Implantation of ICDs (and/or CRT) was an integral component of OMT in all patients, but the decision to implant a device was at the discretion of heart teams at recruiting centers. Device programming was carried out by implanting centers as per usual clinical care.
Clinical follow-up was carried out for a minimum of 24 months, with the last follow-up visit conducted within 3 months of the end of the overall trial follow-up period. Trans-thoracic echocardiography was performed at 6 and 12 months and analyzed by an echocardiography core laboratory at Guy’s and St Thomas’ NHS Foundation Trust, with readers blinded to treatment allocation and the temporal sequence of echocardiograms. Data relating to indication and timing of ICD and CRT insertion aborted sudden death, and sustained ventricular arrhythmias were collected from a dedicated arrhythmia case report form after completion of main trial follow-up using physical and electronic health records at the participating centers, including all scheduled and unscheduled device checks. ICD therapies were classified as appropriate or inappropriate by recruiting centers, as documented in the clinical device interrogation reports; these are the reports used for clinical and governance purposes in the National Health Service of the United Kingdom.
Outcomes
The primary outcome was a composite of all-cause death or aborted sudden death (in turn, defined as an appropriate ICD therapy or a resuscitated cardiac arrest). Secondary outcomes included cardiovascular death or aborted sudden death, appropriate ICD therapy, or sustained ventricular arrhythmia (any ventricular fibrillation or ventricular tachycardia >100 beats per minute that lasts for >30 seconds or requires termination in <30 seconds as a result of hemodynamic compromise9), and total number of appropriate ICD therapies (classified as none, 1, or ≥2 therapies). Outcomes were assessed in all patients over a minimum follow-up period of 24 months, except when sustained ventricular arrhythmia was a component when it was restricted to patients who had an ICD or CRT device in situ, either at randomization or inserted during trial follow-up. The cause of death was adjudicated by an independent blinded clinical events committee; ICD-related outcomes were site reported. All outcomes are defined in Table S1.
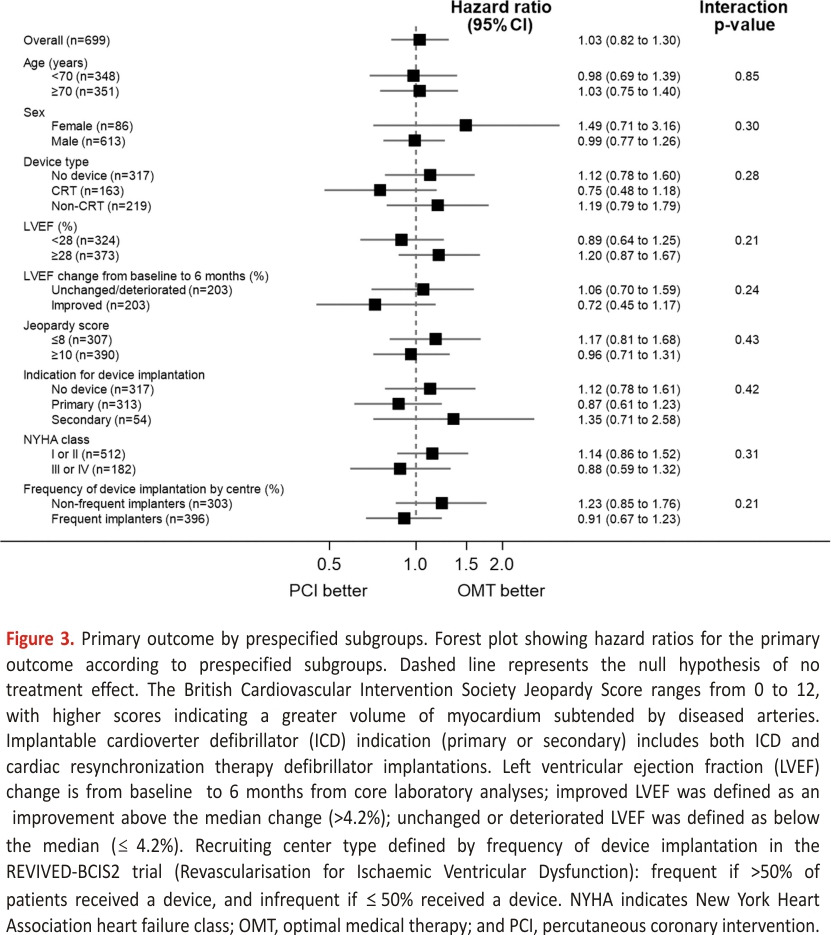
Analyses of the primary outcome were performed in these prespecified subgroups: device type (no device, CRT, non-CRT) indication for ICD (primary or secondary prevention), baseline left ventricular function, improvement in left ventricular function from baseline to 6 months (stratified by median change), extent of coronary artery disease, New York Heart Association functional class, and the type of recruiting center (dichotomized as centers that frequently/infrequently implanted ICDs or CRT devices in trial patients).
Statistical Methods
Because this is a secondary analysis of a trial that has already been completed, a power calculation was not undertaken a priori. For illustration, if 40% of participants experience a primary outcome event over the entire duration of follow-up, equating to ≈250 events, the study would have at least 90% power to detect a hazard ratio of 0.65 or at least 80% power to detect a hazard ratio of 0.70, at a significance level of 5%.10
All analyses were performed on an intention-to-treat basis according to randomized assignment unless otherwise specified. The treatment groups were compared with a Cox proportional hazards model to calculate hazard ratios and 95% CIs. Unadjusted analyses were performed on the primary outcome, with time to the first event measured from randomization to the date of death, withdrawal from the trial, or final trial follow-up. Adjusted analyses were also performed to account for variable timing of ICD or CRT implantation from randomization by including implantation as a time-varying covariate. Sensitivity analyses were performed on the primary outcome in the prespecified subgroup of patients who had an ICD or CRT device at randomization or received one within the first 90 days.
The P value for the treatment difference was calculated with a likelihood ratio test. The proportionality assumption underlying the Cox model was assessed with Nelson-Aalen plots by treatment group. Cumulative event rates were calculated and presented with Kaplan-Meier time-to-event curves. All analyses were undertaken with STATA software, version 17 (Stata Corp). Categorical demographic data are presented as counts (percentages); continuous data are presented as means (Sds) or medians (interquartile ranges), depending on the normality of distribution.
RESULTS
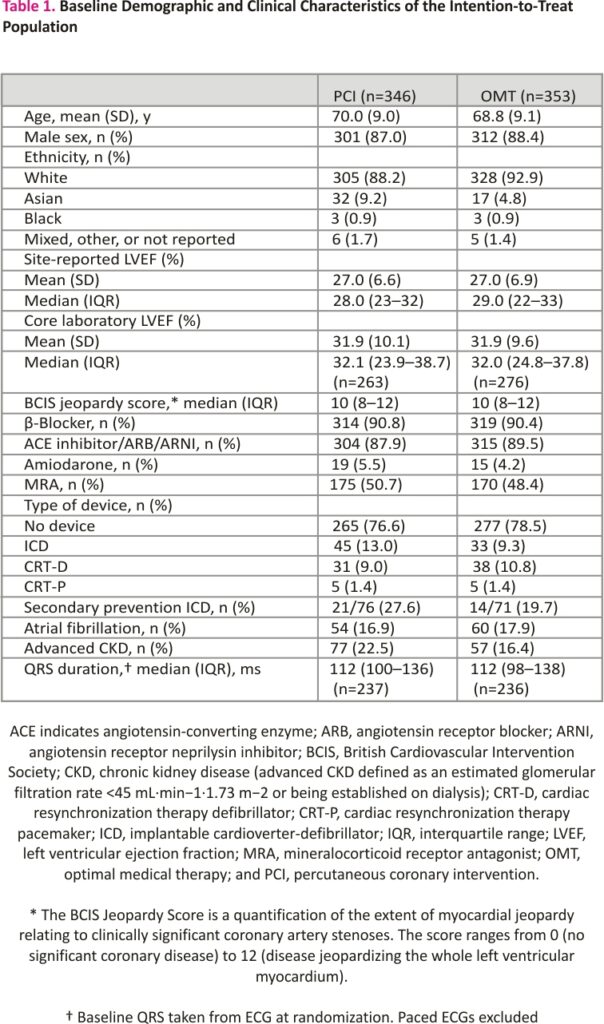
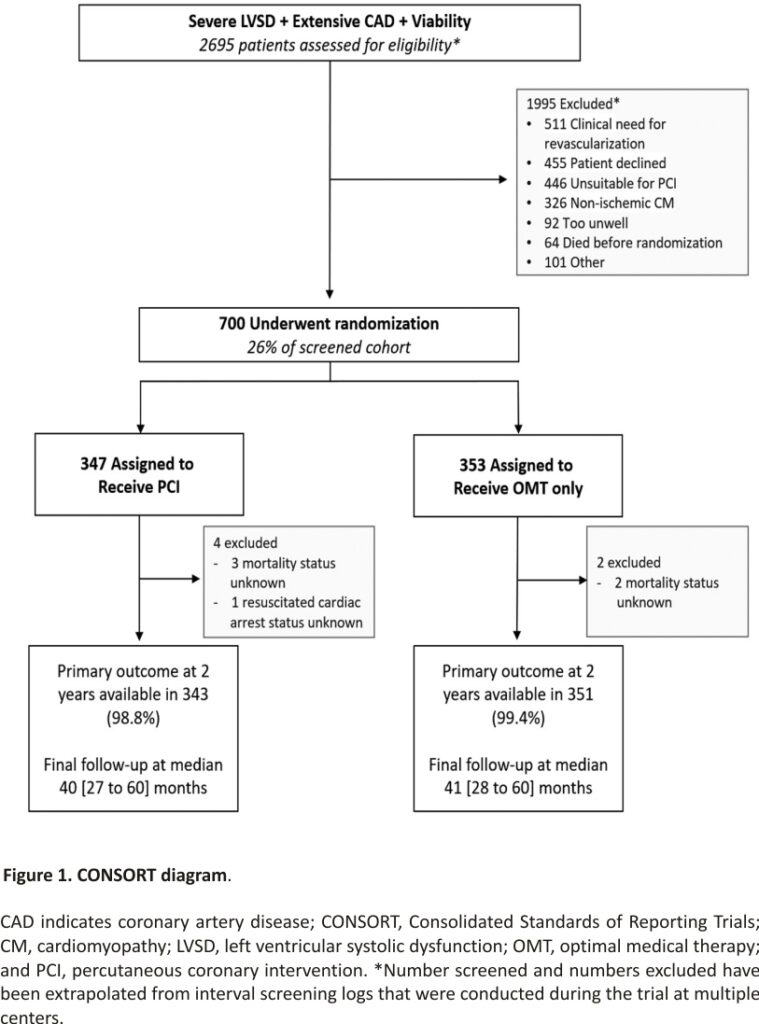
Between August 28, 2013, and March 19, 2020, 700 participants were enrolled; 347 were assigned to the PCI group and 353 to the OMT group (Figure 1). Primary outcome data at 2 years were available for 694 participants, with a median follow-up duration of 41 (27–60) months. Among the patients assigned to the PCI group, 334 (96.3%) underwent PCI at a median of 35 (15–57) days after randomization. In the OMT arm, 10.5% of patients underwent clinical events committee adjudicated unplanned revascularization compared with 2.9% in the PCI arm. Detailed descriptions of the baseline assessments of left ventricular function and viability, titration of OMT, extent of coronary artery disease, and PCI procedural success have been reported previously.7 Baseline clinical and demographic characteristics were evenly distributed between the groups (Table 1).
Primary Outcome
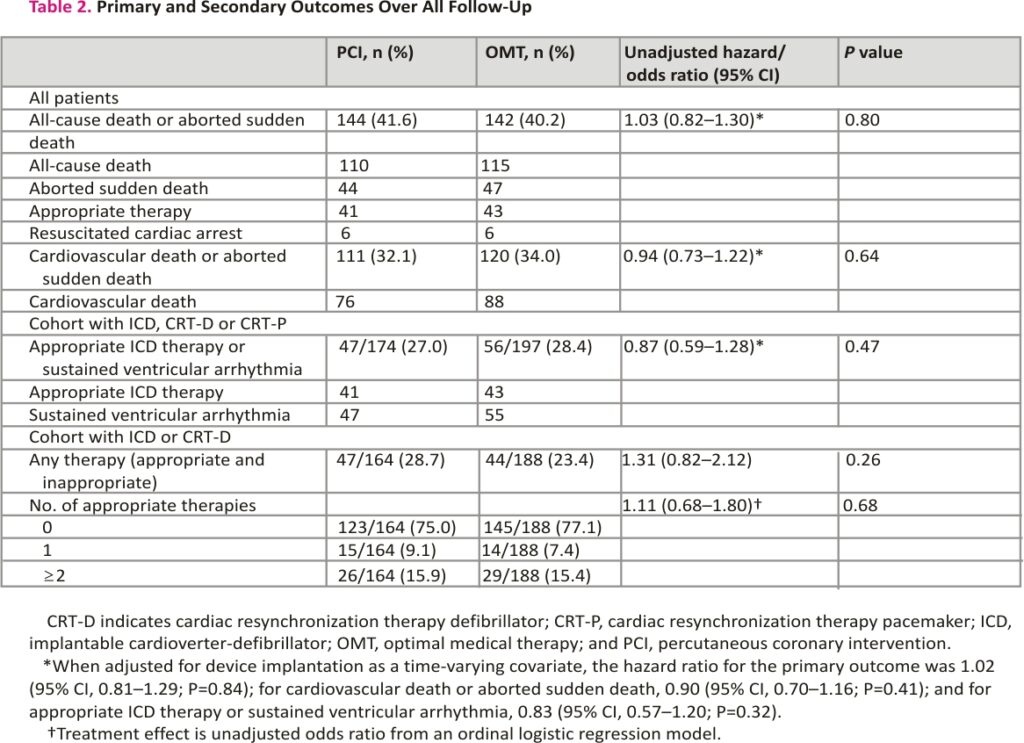
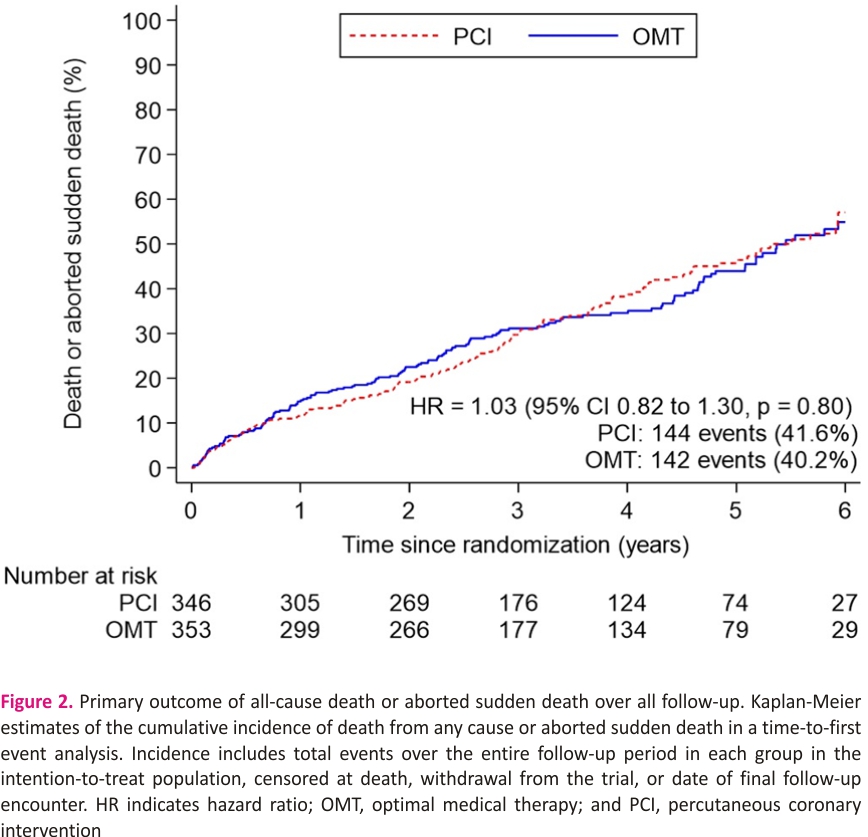
The primary outcome of all-cause death or aborted sudden death occurred in 144 patients (41.6%) in the PCI group and 142 patients (40.2%) within the OMT group (unadjusted hazard ratio, 1.03 [95% CI, 0.82–1.30]; P=0.80); adjusted hazard ratio, 1.02 [95% CI, 0.81– 1.29]; P=0.84; Figure 2). There were 110 deaths (31.8%) in the PCI group and 115 (32.6%) in the OMT group. Aborted sudden death occurred in 44 patients (12.7%) in the PCI group and 47 patients (13.3%) in the OMT group (Table 2). The treatment effect for the primary outcome was consistent across all prespecified subgroups (Figure 3).
Secondary Outcomes
The secondary outcome of cardiovascular death or aborted sudden death occurred in 111 of 346 patients in the PCI group and 120 of 353 in the OMT group (32.1% versus 34.0%; hazard ratio, 0.94 [95% CI, 0.73–1.22]). The composite of appropriate therapy or sustained ventricular arrhythmia occurred in 47 of 174 patients in the PCI group (27.0%) and 56 of 197 patients in the OMT group (28.4%; hazard ratio, 0.87 [95% CI, 0.59–1.28]; P=0.47). There was no between group difference in the total number of appropriate ICD therapies (odds ratio, 1.11 [95% CI, 0.68–1.80]; P=0.68; Table 2).
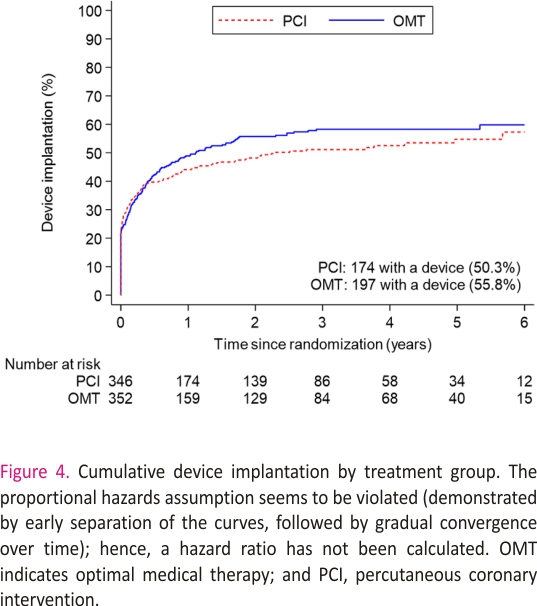
A total of 371 patients (53.1%) had an ICD or CRT device inserted before randomization or during follow-up: 174 of 347 (50.3%) in the PCI group and 197 of 353 (55.8%) n the OMT group (difference, −5.5% [95% CI, −12.9% to 1.9%]; Figure 4 and Table S2). The sensitivity analyses restricted to patients who had an ICD or CRT device in situ at randomization or received one within 90 days yielded results similar to those of the main analyses for the primary outcome (58 [47.2%] versus 62 [50.8%]; hazard ratio, 0.82 [95% CI, 0.57–1.18]; P=0.29) and the secondary outcomes (Tables S3 and S4).
DISCUSSION
In this analysis of the REVIVED-BCIS2 trial, a strategy of PCI in addition to OMT was not associated with a reduction in all cause mortality or aborted sudden death in patients with ischemic left ventricular dysfunction. Furthermore, PCI was not associated with a reduction in the incidence of sustained ventricular arrhythmias or appropriate ICD therapies. The results suggest that patients with stable ischemic left ventricular dysfunction who are on guideline directed medical therapy should not undergo PCI with the sole aim of reducing potentially fatal arrhythmias. The findings also challenge the widespread practice of undertaking CABG or PCI in most patients who are candidates for ICD implantation11 to reduce the arrhythmic risk, an effect believed be mediated by an improvement in left ventricular function. The absence of incremental improvement in left ventricular function in this cohort as a whole7 may also partly explain why PCI was not associated with a reduction in death or aborted sudden death in our study. The 2021 American College of Cardiology/American Heart Association guidelines recommend that revascularization should not be undertaken with the sole purpose of preventing recurrent sustained monomorphic ventricular tachycardia that is suspected to be related to scar.6,12 Our findings now extend this recommendation to the larger group of patients in whom ICD implantation is considered on primary prevention grounds.
In the preliminary report of the REVIVED-BCIS2 trial, appropriate ICD therapies tended to be less frequently observed at 2 years in patients assigned to have PCI, in the subgroup who had a device implanted at baseline or within the first 90 days.7 The current report extends arrhythmia follow-up of this subgroup and reveals that, when the entire duration of the trial is considered and competing mortality is accounted for, PCI is not associated with a reduction in aborted sudden death. This is consistent with the occurrence of the primary outcome in the entire trial cohort, which was not different between treatment arms. The recruited population was younger than the median age of patients newly diagnosed with heart failure in the United Kingdom13 but older than in other randomized trials in this field (mean ages: MADIT II [Multicenter Automatic Defibrillator Implantation 2 Trial], 64 years; MUSTT [Multicenter Unsustained Tachycardia Trial], 67 years; PROTECT-II [Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon.
Pump [IABP] in Patients Undergoing Non Emergent High-Risk PCI], 68 years; DAPA HF [Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial], 66 years; CABG PATCH [Coronary Artery Bypass Graft Patch], 63 years; DANISH [Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality], 63 years; STICH [Surgical Treatment for Ischemic Heart Failure Trial], 59 years; REVIVED-BCIS 2, 70 years). 14–20
To the best of our knowledge, ours is the first trial to assess the effect of randomized allocation to PCI on arrhythmic risk in patients with stable ischemic left ventricular dysfunction. In the CABG PATCH trial, patients with ischemic left ventricular dysfunction and no history of ventricular arrhythmia who were undergoing CABG were randomized to receive either a surgically implanted ICD or no ICD; at an average follow-up of 32 months, there was no significant difference in all-cause mortality between groups.18 The authors hypothesized that the provision of CABG in both arms of the trial reduced the incidence of ventricular arrhythmias to the extent that the incremental benefit of ICD implantation was lost, but they were not able to confirm or refute this hypothesis because there was no medical therapy arm in that trial. In the STICH trial, which randomized patients with ischemic left ventricular dysfunction to either CABG or OMT, there was a significant reduction in the occurrence of sudden death in the CABG group.21 There was, however, a low rate of ICD or CRT implantation. Data on nonfatal ventricular arrhythmias were not captured, and the authors were unable to distinguish between sudden deaths due to ventricular arrhythmia from those caused by acute myocardial infarction or other causes.22,23
Site reported completeness of revascularization in the REVIVED-BCIS2 trial was high, which would be expected to appreciably reduce the burden of inducible ischemia, particularly because the protocol recommended revascularization of all diseased vessels subtending viable myocardium. In this context, the inability to reduce all-cause mortality or aborted sudden death with PCI may indicate that ischemia is less important in the genesis of ventricular arrhythmias than scar, although the relative importance of these 2 factors cannot be definitively discerned without information on the burden and distribution of each. Furthermore, we cannot discount the possibility that PCI may cause periprocedural infarction, which could partially offset the benefit of reducing ischemic burden.24,25 Core laboratory reported angiographic data are pending and may also provide further insights.
Our findings support those of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) investigators, who found no difference in all-cause mortality between patients with documented ischemia who underwent revascularization and those treated with medical therapy alone, although that trial excluded patients with impaired left ventricular function.26
Our study has some limitations. Because more than half of all patients received an ICD or CRT device, the trial has provided important insights into the potential mechanism of death and aborted sudden death in these cases, however, we had less ability to capture nonfatal arrhythmias in patients without devices and may have underestimated the overall event rate. This may have reduced statistical power, but given that the proportions of patients with devices were similar in the 2 trial groups, our estimation of treatment effect is unlikely to have been affected. The number of events detected provides 85% power to detect or exclude a hazard ratio of 0.70, in line with our initial power calculation, although we cannot exclude a smaller treatment effect with PCI. Second, the decision to implant a device was at the discretion of treating physicians and was based on clinical factors and regional differences in policy. Although there was no significant difference in the overall rates of device implantation over the course of the trial, it should be noted that the timing of implantation was not identical in both groups (Figure 4), although analyses adjusted for the timing of implantation and sensitivity analyses of patients with devices in situ at randomization provided conclusions similar to those of the unadjusted analyses. Third, the a priori definition of aborted sudden death included anti-tachycardia pacing or shocks, but we cannot be certain that all ICD therapies were delivered to treat arrhythmias that would have had fatal consequences without such therapies.27,28 Fourth, the protocol did not mandate post-mortem interrogation of ICDs; although this does not affect the primary outcome, it is possible that we may have underestimated the frequency of sustained ventricular arrhythmias in both groups. Last, the classification of appropriate and inappropriate ICD therapy was based on the diagnoses recorded by the clinical teams at recruiting centers, and it is possible that device technicians and electrophysiologists may have been aware of the treatment assignment, so we cannot exclude ascertainment bias.
Conclusions
In patients with ischemic left ventricular dysfunction, a strategy of PCI plus OMT was not associated with a reduction in death or aborted sudden death compared with a strategy of OMT alone. Patients with stable ischemic cardiomyopathy should not undergo PCI solely to reduce the burden of arrhythmias, and in patients who are eligible for an ICD, implantation does not need to be deferred until revascularization has been performed.
Affiliations
National Institute for Health Research Biomedical Research Center and British Heart Foundation Center of Research Excellence at the School of Cardiovascular Medicine and Sciences, King’s College London, United Kingdom (D.P., H.P.M., M.R.). Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom (D.P., C.A.R.). London School of Hygiene & Tropical Medicine, United Kingdom (M.D., T.C., R.E.). Royal Bourne mouth and Christchurch Hospital, Bourne- mouth, United Kingdom (P.D.O.). Leeds Teaching Hospitals NHS Trust and University of Leeds, United Kingdom (J.P.G., M.A.). Belfast Health and Social Care NHS Trust, United Kingdom (S.J.W.). Barts Health NHS Trust, London, United Kingdom (R.W.). Newcastle Hospitals NHS Foundation Trust, United Kingdom (A.M.). King’s College Hospital NHS Foundation Trust, London, United Kingdom (G.A.-Y.). University Hospitals Bristol NHS Foundation Trust, United Kingdom (J.S.). University Hospitals of Leicester NHS Trust, United Kingdom (B.M.). Royal Free Hospital, London, United Kingdom (T.L.). Kettering General Hospital, Northampton, United Kingdom (K.H.). Manchester Royal Infirmary, University NHS Foundation Trust, United Kingdom (F.Z.A.). Edinburgh Royal Infirmary, United Kingdom (M.B.). Sunderland Royal Hospital, United Kingdom (N.J.). Wythenshawe Hospital, Manchester, United Kingdom (E.A.). Institute of Cardiovascular and Medical Sciences, University of Glasgow, United Kingdom (S.W., M.C.P.).
Sources of Funding
The trial was sponsored by King’s College London and funded by the National Institute for Health and Care Research Health Technology Assessment Program. The arrhythmia analyses were supported by the British Heart Foundation (fellow-ship FS/CRTF/21/24190 and the King’s British Heart Foundation Center of Research Excellence grant RE/18/2/34213).
Disclosures
None.
Supplemental Material
REVIVED-BCIS2 sites and investigators Trial organization and oversight Tables S1–S4REVIVED-BCIS2 Arrhythmia Study statistical analysis plan
REFERENCES
1. Gräni C, Benz DC, Gupta S, Windecker S, Kwong RY. Sudden cardiac death in ischemic heart disease: from imaging arrhythmo- genic substrate to guiding therapies. JACC Cardiovasc Imaging. 2020;13: 2223–2238. doi: 10.1016/j. jcmg. 2019.10.021
2. Ryan M, Morgan H, Petrie MC, Perera D. Coronary revascularisation in patients with ischaemic cardiomyopathy. Heart. 2021;107: 612– 618. doi: 10.1136/heartjnl-2020-316856
3. National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure. Technology appraisal guidance [Ta314]. June 25, 2014. Accessed May 6, 2023. https://www.nice. org.UK/guidance/ta314
4. Canty JM, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. CircRes. 2004;94:1142–1149. doi: 10.1161/01.RES.0000125 628.57672.CF
5. Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/ AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defi- brillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013; 10:e11–e58. doi: 10.1016/j.hrthm. 2013. 01.008
6. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018; 138:e272–e3 91. doi:10.1161 /CIR.0000000000000549
7. Perera D, Clayton T, O’Kane PD, Greenwood JP, Weerackody R, Ryan M, Morgan HP, Dodd M, Evans R, Canter R, et al; REVIVED-BCIS2 Investigators. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387:1351– 1360. doi:10.1056/NEJMoa22 06606
8. Perera D, Clayton T, Petrie MC, Greenwood JP, O’Kane PD, Evans R, Sculpher M, Mcdonagh T, Gershlick A, de Belder M, et al; REVIVED Investigators. Percutaneous revascularization for ischemic ventricular dysfunction: rationale and design of the REVIVED-BCIS2 trial: percutaneous coronary intervention for ischemic cardiomyopathy. JACC Heart Fail. 2018; 6:517 –526. doi: 10.1016/j.jchf. 2018.01.024
9. Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, et al; American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/ AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). ACC/AHA/ HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology /American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation. 2006; 114:2534–2570. doi: 10.1161/ CIRCULATIONAHA.106.180199
10. Pocock SJ, Clayton TC, Stone GW. Design of major randomized trials: part 3 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol. 2015;66:2757 –2766. doi: 10.1016/j.jacc. 2015.10.036
11. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi:10.1093/eurheartj/ehy 394
12. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145: e18–e 114. doi:10.1161/CIR.00000000000 01038
13. NICOR. National Heart Failure Audit (NHFA): 2020 summary report (2018/19 Data). Healthcare Quality Improvement Partnership. June 5, 2020. Accessed 6 May 6, 2023.https: //www.nicor.org.uk/ wp-content/up-loads/2020/12/ National-Heart-Failure-Audit-2020-FINAL.pdf
14. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877– 883. doi: 10.1056/NEJMoa 013474
15. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease: Multicenter Unsustained Tachycardia Trial investigators. N EnglJMed. 1999;341:1882–1890. doi: 10.1056/NEJM 1999121634 12503
16. O’Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Dzavík V, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus Intra aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126: 1717–1727. doi: 10. 1161/CIRCULATIONAHA.112.09 8194
17. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa191130 3
18. Bigger JT. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery: Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569–1575. doi:10. 1056/NEJM19971127 3372201
19. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608 029
20. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al; STICH Investigators. Coronary artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011; 364:1607–1616.doi: 10.1056/ NEJMoa 1100356
21. Carson P, Wertheimer J, Miller A, O’Connor CM, Pina IL, Selzman C, Sueta C, She L, Greene D, Lee KL, et al; STICH Investigators. The STICH trial (Surgical Treatment for Ischemic Heart Failure): mode-of-death results. JACC Heart Fail. 2013; 1:400–408. doi:10.1016/j. jchf. 2013.04.012
22. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95: 754–763.doi: 10.1161/01.RES.0000145047. 14691.db
23. John RM, Tedrow UB, Koplan BA, Albert CM, Epstein LM, Sweeney MO, Miller AL, Michaud GF, Stevenson WG. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012; 380:1520–1529. doi:10.1016/S0 140-6736(12)61413-5
24. Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069– 2076. doi: 10.1161/CIRCULATION AHA.109.852517
25. Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi:10. 1161/hc2301.092121
26. Hochman JS, Anthopolos R, Reynolds HR, Bangalore S, Xu Y, O’Brien SM, Mavromichalis S, Chang M, Contreras A, Rosenberg Y, et al. Survival after invasive or conservative management of stable coronary disease. Circulation. 2023;147: 8–19. doi: 10.1161/CIRCULATION AHA.122.062714
27. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, Greenberg H, Hall WJ, Huang DT, et al; MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107
28. Schuger CD, Ando K, Cantillon DJ, Lambiase PD, Mont L, Joung BY, Peress D, Yong P, Wold N, Daubert JP. Assessment of primary prevention patients receiving an ICD: systematic evaluation of ATP: APPRAISE ATP. Heart Rhythm O2.2021;2:405– 411. doi: 10.1016/j.hroo.2021. 07.003
Credit: https://doi.org/10.1161/ CIRCULATIONAHA.123.065300