Lennart Lenk, Michela Carlet, Fotini Vogiatzi, Lea Spory, Dorothee Winterberg, Antony Cousins, Michaela Vossen-Gajcy, Olta Ibruli, Christian Vokuhl, Gunnar Cario, Omar El Ayoubi, Lisa Kramer, Matthias Ritgen, Monika Brüggemann, Robert Häsler, Martin Schrappe, Stephan Fuhrmann, Christina Halsey, Irmela Jeremias, Elias Hobeika, Hassan Jumaa, Ameera Alsadeq & Denis M. Schewe
Abstract
Central nervous system (CNS) involvement remains a challenge in the diagnosis and treatment of acute lymphoblastic leukaemia (ALL). In this study, we identify CD79a (also known as Igα), a signalling component of the preB cell receptor (preBCR), to be associated with CNS-infiltration and – relapse in B-cell precursor (BCP)-ALL patients. Furthermore, we show that downregulation of CD79a hampers the engraftment of leukaemia cells in different murine xenograft models, particularly in the CNS.
Introduction
CNS-involvement is routinely assessed by microscopy of the cerebrospinal fluid (CSF)1, an approach that is limited in performance and informative value. Irrespective of the initial CNS status, all patients are treated with intrathecal chemotherapy which can be neurotoxic2. Hence, besides conclusive diagnostic markers, novel targets for specific eradication of leukaemia cells in the CNS have to be established. In B-cell precursor (BCP)-ALL, certain cytogenetic alterations such as the t(1;19) translocation leading to the E2A-PBX1 fusion and the t(9;22) translocation causing the BCR-ABL fusion (Philadelphia-chromosome) are associated with a higher incidence of CNS-leukemia1,3,4. Recently we have reported that ZAP70 and the Interleukin-7 receptor (IL7R) are associated with CNS-involvement in BCP-ALL5,6. Both proteins are closely associated with the preBCR signalling pathway. The preBCR is composed of the heavy chain (µ), the surrogate light chain (VpreB and λ5) and the signalling components CD79a (Igα) and CD79b (Igβ)7. Activation of the preBCR results in the phosphorylation of tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAMs) of the cytoplasmic tails of Cd79a/ CD79b. This is followed by subsequent recruitment and activation of Src homology kinases, such as LYN and FYN as well as spleen tyrosine kinase (SYK) and ZAP708. Evidence suggesting that the preBCR is involved in the pathogenesis of lymphoid malignancies is accumulating9. Hence, we hypothesized that the preBCR-signaling complex itself is important for CNS-infiltration.
Results and discussion
Cd79a has independent prognostic relevance for CNS-involvement and CNS-relapse in BCP-ALL patients
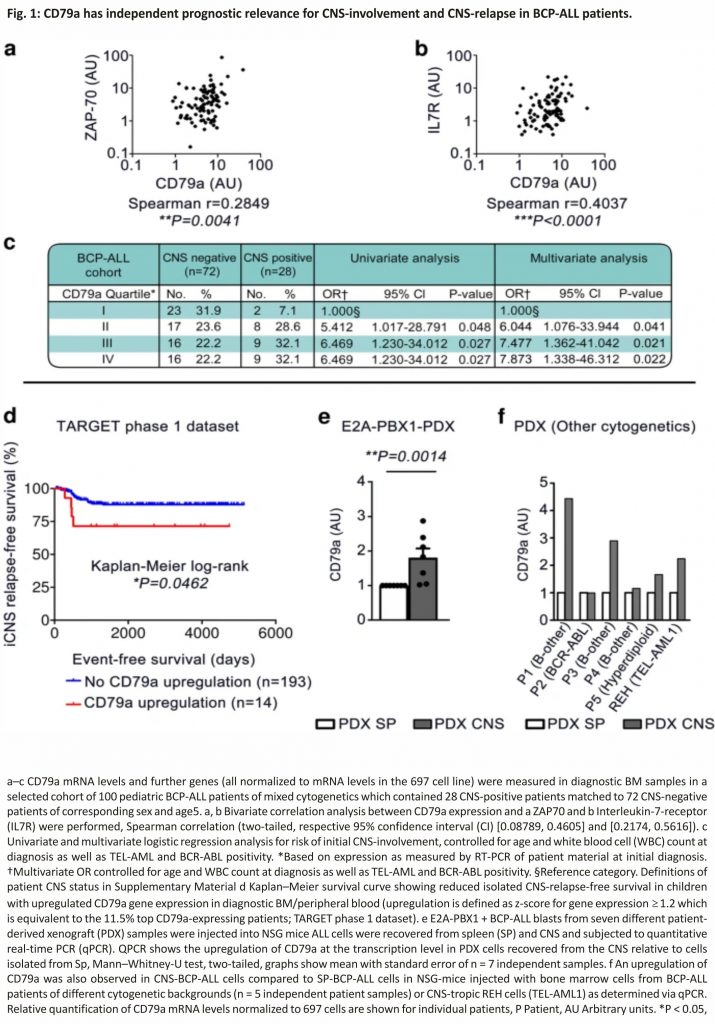
To investigate this, we first measured CD79a mRNA levels in diagnostic bone marrow (BM) samples (70–99.5% blasts), in a cohort of 100 pediatric BCP-ALL patients5. This cohort was selectively designed to contain a high number of CNS-positive patients as only 3–5% of patients are CNS-positive upon initial diagnosis and CNS involvement is thus a rare event1,10. We found that CD79a levels significantly correlated with ZAP70 and with IL7R mRNA levels in bivariate correlation analyses (Fig. 1a, b). Furthermore, we found that patients diagnosed as CNS-positive showed significantly higher levels of CD79a than patients diagnosed as CNS-negative (median CD79a expression: 5.183 ± 0.396 in CNS− vs. 7.537 ± 1.278 in CNS+; Supplementary Fig. 1a, logarithmic scale). Importantly, multivariate logistic regression analysis, excluding the effect of known parameters associated with CNS-infiltration, showed that a CD79a expression above the 75th percentile was associated with a significant ~8-fold increased risk for CNS-positivity compared to the lower quartile (odds ratio = 7.873, 95% confidence interval (CI) [1.338,46.312], p = 0.022, Fig. 1c). CD79a expression levels above the 25th and 50th percentiles were also linked with CNS-involvement (Fig. 1c). Multivariate logistic regression analysis comparing the lower quartile to the second, third and fourth quartiles in CD79a expression revealed a significant 7-fold increased risk for CNS-positivity for patients with CD79a expression levels above the 25th percentile (odds ratio = 7.0, 95% CI [1.4,33.9], p = 0.016, Supplementary Fig. 1b) suggesting that CD79a up-regulation increases the risk for CNS-positivity.
Next, we investigated the association between CD79a and CNS-relapse in an independent cohort from the TARGET phase 1 data set containing gene expression data of more than 200 BCP-ALL cases. We found that an increased expression of CD79a (z-score ≥ 1.2) was associated with significantly reduced long-term probability rates for isolated-CNS-relapse-free survival (Fig. 1d). When analyzing all relapse cases associated with the CNS (combined and isolated), we found that high CD79 levels are associated with a tendency towards reduced CNS-relapse-free-survival (Supplementary Fig. 1c). We found no significant difference for BM-relapse-free probability rates between CD79a high and CD79a low patients further suggesting that CD79a is particularly important for CNS-relapse (Supplementary Fig. 1d).
We then asked if ALL cells retrieved from the CNS show elevated CD79a expression levels. For this approach we xenotransplanted NOD.Cg-PrkdcscidIl2rg tm1Wjl/SzJ (NSG) with diagnostic BM samples of seven different E2A-PBX+ patients (Supplementary Table 1, patients 1–7) and recovered PDX-ALL cells from different organs.
In line with previous reports11, PDX animals exposed some degree of meningeal engraftment, supporting the view that most ALL cells are in principle capable of invading the CNS. Indeed, we found a significant upregulation of CD79a in ALL cells from the CNS as compared to spleen PDX cells (Fig. 1e). Moreover, we found increased levels of CD79a in CNS-ALL cells compared to SP-ALL cells in 4/5 mice transplanted with PDX samples of further BCP-ALL subtypes (3דB-other”, 1×BCR-ABL+, 1×hyperdiploid, Supplementary Table 1, patients 8–12) as well as in a mouse transplanted with the highly CNS-tropic TEL-AML1+ cell line REH (Fig. 1f).
Taken together, our data show that high expression levels of CD79a are associated with CNS-involvement in BCP-ALL patients of different cytogenetics indicating that CD79a, in conjunction with other markers, could be used as a surrogate marker for the assessment of CNS-involvement in BCP-ALL. Furthermore, our data imply that CD79a is upregulated in ALL cells from the CNS in BCP-ALL xenograft models. Moreover, we observed that CD79a mRNA levels strongly correlate with mRNA levels of CD79b in our BCP-ALL patient cohort (Supplementary Fig. 1e) and that CD79b is upregulated in ALL cells recovered from the CNS versus SP from NSG-mice bearing E2A-PBX1-PDX cells (Supplementary Table 1, patients 1–7, Supplementary Fig. 1f) indicating that CD79b is also important for CNS involvement in BCP-ALL.
Cd79a is required for leukemic engraftment particularly in the CNS
Previous reports suggest that some BCP-ALL subtypes, e.g. E2A-PBX1+ BCP-ALL critically depend on preBCR signalling and that others, including BCR-ABL+ BCP-ALL, progress irrespectively of a functional preBCR and are considered as preBCR negative12.
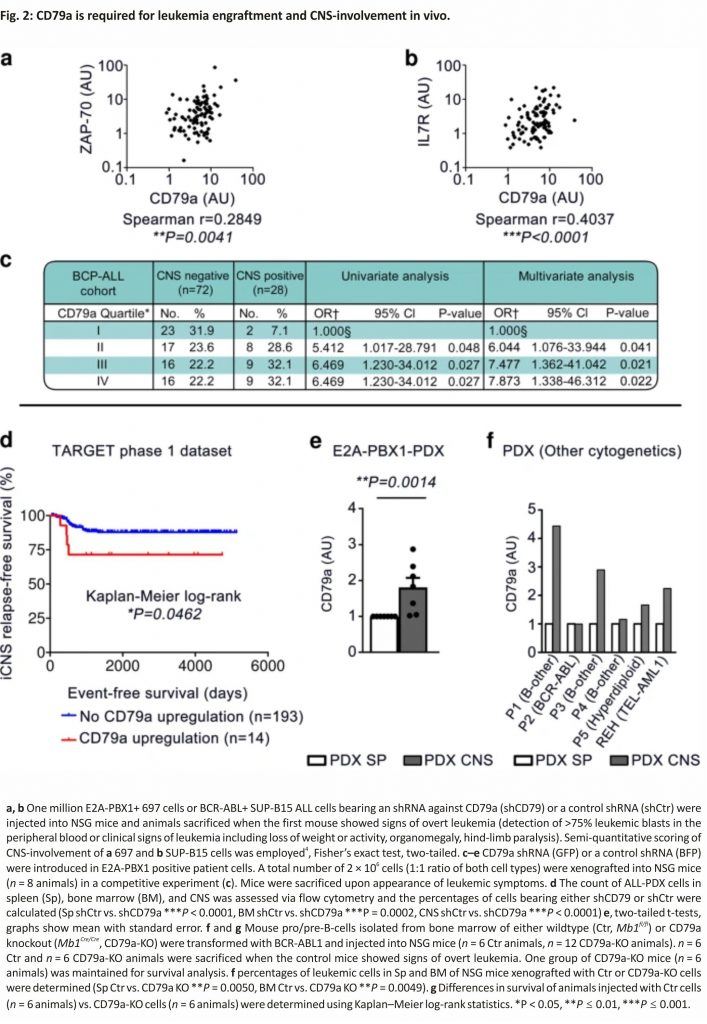
To further investigate this issue and the role of CD79a in CNS involvement in vivo we utilized two cell lines 697 (E2A-PBX1+) and SUP-B15 (BCR-ABL+) representing preBCR-positive and preBCR-negative BCP-ALL cells. First, we performed short-hairpin RNA-interference-mediated knockdown of CD79a in the human cell lines (Supplementary Fig. 2a). Control cells (carrying shRNA against a target sequence from Renilla spp., shCtr) and cells carrying CD79a shRNA (shCD79a) were injected into NSG mice and leukaemia engraftment in different organs was analyzed. Indeed, animals injected with 697-shCD79a cells exposed a significant reduction of leukemic burden in the CNS (4/15 CNS+ animals, 26%) as compared to animals injected with control cells (7/10 CNS+ animals, 70%) (Fig. 2a). In contrast, leukemic infiltration in spleen and BM, as well as survival times, were comparable between both groups (Supplementary Fig. 2b–d). SUP-B15-shCD79a cells xenografted into NSG mice showed similar engraftment in the spleens and BMs as compared to SUP-B15-shCtrl cells (Supplementary Fig. 2e, f). However, animals injected with SUP-B15-shCD79a cells only showed weak infiltration of the leptomeninges whereas SUP-B15-shCtrl cells caused pronounced and multi-layered CNS-leukemia (Fig. 2b). Furthermore, animals injected with SUP-B15-shCD79a cells exposed a small but significant prolongation of median mouse survival by 12 days (Supplementary Fig. 2g), which is most likely due to the delay in the development of CNS-leukemia. Together these data indicate an important role of CD79a for the engraftment of E2A-PBX1+ (preBCR-positive) and BCR-ABL+ (preBCR-negative) BCP-ALL cells in that niche.
To further substantiate the role of CD79a in CNS-involvement, CD79a knockdown experiments were performed using BCP-ALL PDX primary cells. PDX cells from an E2A-PBX1+ patient (Supplementary Table 1, patient 5) were stably transduced with lentiviral constructs harbouring either a blue fluorescent protein (BFP) or a green fluorescent protein (GFP) reporter gene cassette. BFP cells were then stably transduced with a second vector which carried a dsRED-reporter gene coupled to the control shRNA described above (PDX-shCtr), whereas GFP cells were transduced with dsRED-reporter gene fused with a CD79a shRNA (PDX-shCD79a). Both cell types were then injected into NSG mice in a 1:1 ratio to study whether CD79a provides a niche-specific engraftment advantage (Fig. 2c, Supplementary Fig. 3a, b). Indeed, upon clinical signs of overt leukaemia, significant variations in the percentage of engrafted populations of PDX-shCD79a compared to PDX-shCtr were observed in the spleen (12% vs. 88%), the BM (28% vs. 72%), and the CNS (2% vs. 98%) (Fig. 2d). Of note, the proportion of PDX-shCD79a to PDX-shCtr cells was significantly lower in PDX cells recovered from the CNS as compared to other organs (Fig. 2d, e) indicating an important role for CD79a for leukaemia engraftment in mice, particularly in the CNS.
The role of CD79a in BCR-ABL+ ALL was further analyzed using a murine/ murine transplantation model. BM cells were isolated from mice carrying either a LoxP-flanked variant of the Mb1-wildtype gene, which codes for CD79a (Mb1fl/fl; CD79a-Ctrl) or from CD79a knock-out mice (Mb1Cre/Cre; CD79a-KO)13 (Supplementary Fig. 4a). Cells were cultured for 3 days in the presence of IL7, an essential growth factor for early B-cells14. Subsequently, cells were transduced with a retroviral vector for BCR-ABL1 expression and cultured independent of IL7 to ensure malignant transformation15. CD79a-KO cells proliferated at a similar rate in vitro as compared to control (Supplementary Fig. 4b). Cells were then injected into mice. Transformed cells led to overt leukaemia in mice within 29 days (exemplary BM smear in Supplementary Fig. 4c). In contrast, we found that animals injected with BCR-ABL1+ pro-B-cells lacking CD79a exhibited a significant delay in leukemic engraftment in the spleen, BM, and CNS (Fig. 2f, Supplementary Fig. 4d, e). No animal of the CD79a-KO group sacrificed on day 29 displayed signs of CNS-infiltration (Supplementary Fig. 4e). Importantly, animals injected with CD79a-KO cells showed a highly significant median survival prolongation of 66 days compared to mice injected with CD79a-Ctr cells (29 days vs. 95 days, Fig. 2g). These results suggest that whereas low expression levels of CD79a result in an engraftment disadvantage in the CNS, complete absence of CD79a impacts the overall engraftment of BCP-ALL cells in vivo. These findings are intriguing as previous studies have underestimated the role of preBCR signalling in BCP-ALL, particularly for subgroups which are considered preBCR negative, as they lack an assembled preBCR complex on the surface, such as BCR-ABL+ ALL12. Here we provide evidence that the signalling molecules of the preBCR are indispensable for leukaemia development and CNS involvement. Supporting this view, ZAP70, PI3K, and MAPK pathways, which all act downstream the preBCR were shown to be directly involved in CNS involvement5,16,17. Furthermore, molecules associated with the adherence to vascular and conjunctive tissue in the meningeal microenvironment such as α6 integrin and VEGF were reported to promote CNS infiltration and survival in the CNS niche16,18. Hence, we assume that molecules highly abundant in the meningeal microenvironment interact with ALL cells and promote adherence and survival signalling in the CNS by crosstalk with the preBCR-signaling complex10. Yet, our results do not necessarily apply to the relapse situation, as we did not find increased CD79a levels in samples obtained at CNS relapse when analyzing six matched pairs of diagnostic BM samples and CNS-relapse samples in our BCP-ALL cohort. So far, small molecule inhibitors were used to regulate preBCR downstream signaling12,16,19. Our study promotes the view that one could also modulate this pathway by targeting the receptor itself. In this regard, IgM and CD79b antibodies, the latter being used in lymphoma treatment, may be effective options20. We found that not only CD79a but also its dimerization partner CD79b is upregulated in PDX-ALL cells isolated from the CNS of xenotransplanted mice. Accordingly, prospective measurements of CD79a and CD79b inpatient samples will help to validate the role of these molecules in CNS involvement in BCP-ALL.
Overall, using different pre-clinical mouse models of BCP-ALL, we show that the absence of the preBCR signalling unit CD79a is associated with an attenuation of leukemic infiltration, particularly in the CNS. Hence, CD79a may represent a promising target for novel diagnosis and treatment approaches in CNS leukaemia.
Methods
Patient samples and human cell lines
BCP-ALL patients were treated according to ALL-Berlin-Frankfurt-Münster (BFM) 2000 or 2009 protocols after informed consent in accordance with the Declaration of Helsinki. Our study was approved by the ethical committee of the Christian-Albrechts-University Kiel (D437/17). 697, SUP-B15, and REH cells were obtained from DSMZ. Authentication of cell lines was performed using flow cytometry (BCP-ALL markers such as hCD19, hCD45) and RT-PCR was used to confirm the chromosomal translocation for BCR-ABL in SUP-B15 cells. All cell lines were regularly tested for the absence of mycoplasma contamination using the MycoAlert™ Mycoplasma Detection Kit (Lonza).
BCP-ALL xenografts and isolation of PDX cells from different niches
NOD. Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from Charles River and xenografts generated in accordance with governmental regulations (Schleswig-Holstein Ministerium für Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung)4,5,6,21. One million ALL cells were injected intravenously into female NSG-mice (6–10 weeks of age) and leukemic engraftment was followed by detection of human CD45+/murine CD45−/human CD19+ cells in the peripheral blood via flow cytometry analysis. Animals were sacrificed when showing signs of overt leukaemia (detection of >75% leukemic blasts in the peripheral blood or clinical signs of leukaemia including loss of weight or activity, organomegaly, hind-limb paralysis). Leukemic infiltration of the murine CNS was assessed in histological sections in blinded experiments and the scorings CNS− (no CNS infiltration of the leptomeninges), CNS+ (week infiltration of the leptomeninges) and CNS++ (strong and multilayered meningeal infiltration) were discriminated4,6. For the recovery of ALL cells from murine organs, mice were sacrificed by CO2 inhalation and spleens, hind leg bones and heads were collected. BM cells were isolated by flushing out the bones using a 27 G syringe. For extraction of the meninges, mouse skulls were opened, brains extracted and the meninges were carefully detached from the skull with tweezers. Spleen and CNS were homogenized using a 70 µm Nylon cell strainer. Spleen, BM, and CNS cells were subjected to red blood cell lysis, washed with PBS and purity of ALL cells were assessed via flow cytometry detecting human CD45, murine CD45, and human CD19.
Flow cytometry
A minimum of 1 × 106 cells was used for flow cytometry staining. Intracellular flow cytometry staining was performed using the Fixation/Permeabilization Solution Kit (BD Bioscience). Cell viability was measured using Sytox® blue dead cell stain (Life Technologies). A FACS Canto II (BD Biosciences) or a MACSQuant X (Miltenyi Biotec) were used for flow cytometry. FlowJo v.10.7 was used for data analysis. Detailed information on the gating strategy is provided in Supplementary Fig. 5. Antibodies for flow cytometry (hCD19, hCD45, mCD45, and CD79a) were purchased from BioLegend. Detailed information on antibodies used for flow cytometry analysis is provided in Supplementary Table 2
Statistics and reproducibility
Statistical analysis was performed using GraphPad PRISM 5.00, SPSS 22, SigmaPlot 12.5, and/or R v.3.3.3. Gaussian distribution was tested using the Shapiro– Wilk test. Statistical significance was assessed using an unpaired t-test, a Mann–Whitney test or ANOVA, depending on the normal distribution and group numbers. Differences in survival were calculated using Kaplan–Meier log-rank statistics. A P-value of <0.05 was considered significant. The number (n) of independent biological replicates (at least n = 3) are indicated in the figure captions. Associations between gene expression and CNS status were examined by unconditional logistic regression to calculate odds ratios (ORs) and 95% CIs or using Cox proportional Hazards Model5,22. Bivariate correlations were analyzed calculating the Spearman correlation coefficient5.
Gene expression datasets for re-evaluation
For analysis of CNS relapse in patients, the dataset from the United States National Cancer Institute “TARGET phase 1 ALL Project” was used23. Microarray data from diagnostic BM (n = 131 independent patient samples) or peripheral blood (n = 76 independent patient samples) of children with high-risk BCP-ALL annotated with clinical follow-up data including CNS-relapse were thereby analyzed24,25. The dbGaP data is available via the dbGaP accession phs000218.v21.p7. The microarray data were log-transformed and z-scores calculated for CD79a. CD79a upregulation was defined as a z-score for gene expression ≥1.2 (TARGET phase 1 data set). This is equivalent to the top 11.5% CD79a-expressing patients.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary information files (Supplementary Information and Supplementary Data 1). The results published here are in part based upon data generated by the Therapeutically Applicable Research to Generate Effective Treatments (https://ocg.cancer. gov/programs/target) initiative, phs00 0218. The data used for this analysis are available at https://www.ncbi.nlm. nih.gov/projects/gap/cgi-bin/study. cgi?study_id=phs000218.v21.p7 (dbGaP accession: phs000218.v21.p7) managed by the United States National Cancer Institute (NCI). Microarray data used for the analysis are available on [ftp://caftpd.nci.nih.gov/pub/OCG-DCC/TARGET/ALL/clinical/Phase1/; microarray GEO accession GSE11877].
References
1. Pui, C.-H. & Howard, S. C. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 9, 257–268 (2008). Article Google Scholar
2. Cheung, Y. T. et al. Association of cerebrospinal fluid biomarkers of central nervous system injury with neurocognitive and brain imaging outcomes in children receiving chemotherapy for acute lymphoblastic leukaemia. JAMA Oncol. 4, e180089 (2018). Article Google Scholar
3. Jeha, S. et al. Increased risk for CNS relapse in pre-B cell leukaemia with the t(1;19)/TCF3-PBX1. Leukemia 23, 1406–1409 (2009). CAS Article Google Scholar
4. Krause, S. et al. Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukaemia (ALL) in the central nervous system (CNS). Blood 125, 820–830 (2015). CAS Article Google Scholar 5. Alsadeq, A. et al. The role of ZAP70 kinase in acute lymphoblastic leukaemia infiltration into the central nervous system. Haematologica 102, 346–355 (2017). CAS Article Google Scholar
6. Alsadeq, A. et al. IL7R are associated with CNS infiltration and relapse in pediatric B-cell precursor acute lymphoblastic leukaemia. Blood 132, 1614–1617 (2018). CAS Article Google Scholar
7. Hardy, R. R. & Hayakawa, K. B cell development pathways. Annu. Rev. Immunol. 19, 595–621 (2001). CAS Article Google Scholar
8. Herzog, S., Reth, M. & Jumaa, H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat. Rev. Immunol. 9, 195–205 (2009).CAS Article Google Scholar
9. Rickert, R. C. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat. Rev. Immunol. 13, 578–591 (2013). CAS Article Google Scholar
10. Lenk, L., Alsadeq, A. & Schewe, D. M. Involvement of the central nervous system in acute lymphoblastic leukaemia. Opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 39, 173–187 (2020). Article Google Scholar
11. Williams, M. T. S. et al. The ability to cross the blood-cerebrospinal fluid barrier is a generic property of acute lymphoblastic leukaemia blasts. Blood 127, 1998–2006 (2016). CAS Article Google Scholar
12. Geng, H. et al. Self-enforcing feedback activation between BCL6 and pre-B cell receptor signalling defines a distinct subtype of acute lymphoblastic leukaemia. Cancer Cell 27, 409–425 (2015). CAS Article Google Scholar
13. Hobeika, E. et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl Acad. Sci. USA 103, 13789–13794 (2006). CAS Article Google Scholar
14. Peschon, J. J. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 (1994). CAS Article Google Scholar
15. Shojaee, S. et al. PTEN opposes negative selection and enables the oncogenic transformation of pre-B cells. Nat. Med. 22, 379–387 (2016). CAS Article Google Scholar
16. Yao, H. et al. Leukaemia hijack a neural mechanism to invade the central nervous system. Nature 560, 55–60 (2018). CAS Article Google Scholar
17. Irving, J. et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukaemia and confer sensitivity to MEK inhibition. Blood 124, 3420–3430 (2014). CAS Article Google Scholar
18. Münch, V. et al. Central nervous system involvement in acute lymphoblastic leukaemia is mediated by the vascular endothelial growth factor. Blood 130, 643–654 (2017). Article Google Scholar
19. Köhrer, S. et al. Pre-BCR signalling in precursor B-cell acute lymphoblastic leukaemia regulates PI3K/AKT, FOXO1 and MYC, and can be targeted by SYK inhibition. Leukaemia 30, 1246– 1254 (2016). ArticleGoogle Scholar
20. Pfeifer, M. et al. Anti-CD22 and anti-CD79B antibody-drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukaemia 29, 1578–1586 (2015). CAS Article Google Scholar
21. Vogiatzi, F. et al. Daratumumab eradicate the minimal residual disease in a preclinical model of pediatric T-cell acute lymphoblastic leukaemia. Blood 134, 713–716 (2019). CAS Article Google Scholar
22. Cario, G. et al. High interleukin-15 expression characterizes childhood acute lymphoblastic leukaemia with the involvement of the CNS. J. Clin. Oncol. 25, 4813–4820 (2007). CAS Article Google Scholar
23. Mullighan, C. G. et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukaemia. New Engl. J. Med. 360, 470–480 (2009). CAS Article Google Scholar
24. Borowitz, M. J. et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukaemia: relation to other risk factors. A Children’s Oncology Group study. Leukaemia 17, 1566–1572 (2003). CAS Article Google Scholar
25. Bowman, W. P. et al. Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukaemia: results of Children’s Oncology Group Trial P9906. Pediatr. Blood Cancer 57, 569–577 (2011) Article Google Scholar
Acknowledgements
D.M.S. is funded by the Deutsche Krebshilfe e.V. (111963), the Wilhelm Sander Stiftung (2016.110.1 and 2019.119.1) and the Deutsche José-Carreras Leukämiestiftung (DJCLS 17R/2017). H.J. is supported by the Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (SFB1074; projects A10, B6) and ERC advanced grant. A.C. is funded by the William and Elizabeth Davies Foundation and C.H. by the CCLG/Little Princess Trust (CCLGA 2017 13). I.J. is funded by grants from the European Research Commission (Consolidator Grant 681524), a Mildred Scheel Professorship by the Deutsche Krebshilfe, the German Research Foundation (DFG) Collaborative Research Center 1243 “Genetic and Epigenetic Evolution of Hematopoietic Neoplasms”, projects A05, DFG proposal MA 1876/13-1, the Bettina Bräu Stiftung and the Dr. Helmut Legerlotz Stiftung. Sequencing, NGS data management and data processing were supported by the CCGA (DFG, INST 257/605-1) as well as by the Cluster of Excellence ExC PMI 2167. E.H. is supported by the Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (SFB1074; projects A9). We thank the patients and physicians who contributed samples and data for this study. We thank Cornelia Eckert for kindly providing relapse patient samples. We thank Katrin Timm-Richert, Katrin Neumann, Birthe Fedders, Annette Tietz, Silvia I wersen, Gabriele Riesen, Steffi Spielberg, Annette Frank, and Fabian Klein for the excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
These authors contributed equally: Ameera Alsadeq, Denis M. Schewe.
Affiliations
Department of Pediatrics I, ALL-BFM Study Group, Christian-Albrechts University Kiel and University Medical Center Schleswig-Holstein, Arnold-Heller-Str. 3, Haus C, 24105, Kiel, Germany
Lennart Lenk, Fotini Vogiatzi, Lea Spory, Dorothee Winterberg, Michaela Vossen-Gajcy, Olta Ibruli, Gunnar Cario, Martin Schrappe & Denis M. Schewe
Research Unit Apoptosis in Hematopoietic Stem Cells, Helmholtz Zentrum München, German Center for Environmental Health (HMGU), Marchioninistraße 25, 81377, Munich, Germany
Michela Carlet & Irmela Jeremias
Institute of Cancer Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Garscube Estate, Switchback Road, Bearsden, Glasgow, G61 1QH, UK
Antony Cousins & Christina Halsey
Department of Pathology, Section of Pediatric Pathology, Venusberg-Campus 1, Gebäude 62, 53127, Bonn, Germany
Christian Vokuhl
Institute of Immunology, Ulm University Medical Center, Albert-Einstein-Allee 11, 89081, Ulm, Germany
Omar El Ayoubi, Lisa Kramer, Elias Hobeika, Hassan Jumaa & Ameera Alsadeq
Department of Medicine II, University Hospital Schleswig-Holstein, Langer Segen 8-10, 24105, Kiel, Germany
Matthias Ritgen & Monika Brüggemann
Institute of Clinical Molecular Biology, Christian-Albrechts University Kiel and University Medical Center Schleswig-Holstein, Campus Kiel, Rosalind-Franklin-Straße 12, 24105, Kiel, Germany
Robert Häsler
Department of Hematology and Oncology, HELIOS Hospital Berlin-Buch, Rosalind-Franklin-Straße 12, 24105, Kiel, Germany
Stephan Fuhrmann
German Cancer Consortium (DKTK), Partnering Site Munich, Pettenkoferstr. 8a, 80336, München, Germany
Irmela Jeremias
Department of Pediatrics, Dr. von Hauner Children’s Hospital, University Hospital, LMU Munich, Lindwurmstraße 4, 80337, München, Germany
Irmela Jeremias
Contributions
L.L. designed and performed experiments, and analyzed data. M.C., F.V., L.S., D.W., O.I., O.E, C.V., L.K., and I.J. performed experiments and analyzed data. G.C., M.R., M.B., S.F., and M.S. provided ALL samples and clinical data. A.C., C.H., M.V.-G., and R.H. provided dataset analyses. E.H. and H.J. provided mouse models and discussed the research direction. A.A. and D.M.S. initiated and designed experiments and discussed the research direction. L.L., A.A., and D.M.S. wrote the manuscript. All authors discussed the manuscript.
Corresponding author
Correspondence to Denis M. Schewe.
Ethics declarations
Competing interests
The authors declare the following competing interests: M.B. received consulting fees from PRMA, research funding from Amgen, honoraria from Novartis, Pfizer, and Amgen and was an advisory board member for Incyte and Amgen. D.M.S. was an advisory board member for Bayer, SOBI and Jazz Pharmaceuticals and received research funding from OSE Pharmaceuticals. M.S. received research funding from the Shire, and from Servier, as well as fees for Advisory Board functions from Jazzpharma, and Servier. The other authors declare no competing interests.
Credits: Lenk, L., Carlet, M., Vogiatzi, F. et al. CD79a promotes CNS-infiltration and leukaemia engraftment in pediatric B-cell precursor acute lymphoblastic leukaemia. Commun Biol 4, 73 (2021). https://doi.org/10.1038/s42003-020-01591-z