Lacin Cevhertas, Ismail Ogulur, Debbie J. Maurer, Daniel Burla, Mei Ding, Kirstin Jansen, Jana Koch, Chengyao Liu, Siyuan Ma, Yasutaka Mitamura, Yaqi Peng, Urszula Radzikowska, Arturo O. Rinaldi, Pattraporn Satitsuksanoa, Anna Globinska, Willem van de Veen, Milena Sokolowska, Katja Baerenfaller, Ya‐dong Gao, Ioana Agache, Mübeccel Akdis, Cezmi A. Akdis
Abstract
In this review, we discuss recent publications on asthma and review the studies that have reported on the different aspects of the prevalence, risk factors and prevention, mechanisms, diagnosis, and treatment of asthma. Many risk and protective factors and molecular mechanisms are involved in the development of asthma. Emerging concepts and challenges in implementing the exposome paradigm and its application in allergic diseases and asthma are reviewed, including genetic and epigenetic factors, microbial dysbiosis, and environmental exposure, particularly to indoor and outdoor substances. The most relevant experimental studies further advancing the understanding of molecular and immune mechanisms with potential new targets for the development of therapeutics are discussed. A reliable diagnosis of asthma, disease endotyping, and monitoring its severity is of great importance in the management of asthma. Correct evaluation and management of asthma comorbidity/multimorbidity, including interaction with asthma phenotypes and its value for the precision medicine approach and validation of predictive biomarkers, are further detailed. Novel approaches and strategies in asthma treatment linked to mechanisms and endotypes of asthma, particularly biologicals, are critically appraised. Finally, due to the recent pandemics and its impact on patient management, we discuss the challenges, relationships, and molecular mechanisms between asthma, allergies, SARS‐CoV‐2, and COVID‐19.
1 INTRODUCTION
Asthma is a chronic heterogeneous disease of the lower airways characterized by chronic inflammation and airway hyperreactivity leading to cough, wheeze, difficulty in breathing, and chest tightness. The pathophysiology of asthma is complex. In the past three decades, a better understanding of distinct asthma visible properties (phenotypes) and mechanisms (endotypes) shaped better diagnostic and therapeutic tools in support of stratified/personalised interventions based on recognition of differences in responsiveness to various therapeutic interventions (therapies).1,2 In addition, environmental factors, genetic polymorphisms, and epigenetic factors contribute to the development of asthma, heterogeneity in phenotyping, and steroid responsiveness.3-6 Environmental interventions and exposure control can improve asthma control and exacerbations.7
The incidence and prevalence of asthma are increasing, though regular use of inhaled corticosteroids (ICS) reduces mortality.8,9 New therapies and therapeutic targets are required for better control of symptoms and exacerbations in severe asthma patients and for avoiding adverse reactions caused by the administration of oral corticosteroids (OCS).
This review highlights the recent studies on immunopathological pathways, molecular mechanisms, various environmental factors, and microbial dysbiosis in asthma. Clinical trials, multi-centred international studies, and real‐world data are reviewed for novel approaches in asthma diagnosis, candidate biomarkers, and management of asthma in adults and children.
2 EPIDEMIOLOGY AND RISK FACTORS IN THE DEVELOPMENT OF ASTHMA
2.1 Asthma prevalence in adults and children
Asthma prevalence seems to be still increasing as suggested by Borna et al., who investigated its change between 2008 and 2016 in Sweden. The authors observed a significant rise of reported frequencies of ever asthma, physician-diagnosed asthma, use of asthma medication, and current asthma, especially in young adults aged 16‐25 years.9 At the same time, an increase in the prevalence of respiratory symptoms during the same period was reported, suggesting the possibility that actually, asthma is underdiagnosed. The potential risk factors for asthma remained the same during the study period.9
Recent studies assessed the prevalence of asthma in preschoolers (4.4%) and elementary school children (6.4%) according to the Global Initiative for Asthma (GINA) definition.8 While no significant difference between rural and urban children was observed, Branco et al10 found an association with previously identified risk factors for asthma development, including parental asthma and antibiotics in the first year of life. Liu et al investigated the link between maternal hypothyroidism in the perinatal period and childhood asthma risk, in a population‐based cohort study using Danish national registers. A higher incidence of asthma was found compared to children born to mothers with no thyroid dysfunction. The risk was even higher if the mothers did not receive thyroid hormone treatment during pregnancy.11
2.2 Gender‐specific differences in prevalence
In a study involving 3115 Swiss adolescents, Ödling et al. reported that asthma tended to be more common among girls compared to boys; however, boys with asthma had more often a doctor’s diagnosis.12 Uncontrolled asthma was more common among girls than boys, who also were more often dispensed with high daily doses of ICS compared to girls. Subjects with persistent early‐onset asthma had more often a doctor’s diagnosis compared to adolescent-onset asthma. The authors highlighted the clinical relevance of monitoring female adolescents with uncontrolled asthma.12 Gender‐specific prevalence of rhinitis and asthma as single and multimorbid diseases during puberty was examined in six European population‐based birth cohorts of MeDALL. Male predominance in prevalence before puberty and the “gender‐shift” toward females after puberty onset were strongest in multimorbid patients who had rhinitis and asthma concurrently.13
3 RECENT DEVELOPMENTS IN ASTHMA‐RELATED GENES
Genetic factors have a strong impact on the risk of developing asthma.2 In particular, childhood asthma is strongly associated with the 17q21 locus alleles. Analyzing 14 different populations of asthmatic patients from 12 different countries, Farzan et al. showed that 17q21 polymorphism is related to an increased risk of exacerbations in children with asthma despite ICS use.14 The authors also observed that the SNP rs7216389 frequency was higher in East Asians, African Americans, and Hispanics, compared to patients of European ancestry.14 Interestingly, an interaction between 17q21 variants and breastfeeding in relation to respiratory symptoms in the first year of life was observed by Gorlanova et al suggesting a protective effect of breastfeeding on asthma inception linked to early‐life respiratory infections in babies carrying the risk alleles. Specifically, when infants were stratified by breastfeeding status, carriers of asthma risk alleles showed a protective effect of breastfeeding on respiratory symptoms during the weeks when they were breastfed, while they showed an increased risk of respiratory symptoms during the weeks when they were not breastfed.15 The ORMDL3 gene, also related to the 17q21 region, plays an important role as well. There are higher levels of human lung ORMDL3 and its SNPs rs8076131 from asthmatic patients than the healthy subjects.16
Associations between childhood asthma phenotypes and genetic, immunological, and environmental factors have been largely established, whereas there is a lack of strategies to integrate high‐dimensional risk factors from multiple distinct datasets and thereby increase the statistical power of analyses.17 In many studies, the classification of childhood asthma phenotypes is often based on the assessment of singular risk factor measurements only. Krautenbacher et al evaluated a new strategy combining cytokine, genotype, flow cytometry, diagnostic, questionnaire, reverse transcription‐polymerase chain reaction (RT‐PCR), and microarray data by using an integrative multilevel learning approach. Following this new discovery approach, genes such as PKN2 (protein kinase N2), PTK2 (protein tyrosine kinase 2), and ALPP (alkaline phosphatase, placental) seem to be the most important asthma risk factors.18
Interleukin 1 receptor‐like 1 (ST2) is known to be related to the pathogenesis of allergic diseases by mediating the response to IL‐33. Interleukin 1 receptor‐like 1 (ST2) single‐nucleotide polymorphisms (SNPs) rs13431828, rs1420101, rs1921622, and rs10204137 were related to reduced efficacy of ICS in children and adolescents.19
Recently, Olafsdottir et al3 demonstrated 88 asthma risk genome variants at 56 loci, 19 previously unreported, in a genome‐wide association meta‐analysis. They investigated the effect of asthma‐associated variants and their genetic correlation between asthma and allergic phenotypes as well. They suggest a missense variant in TNFRSF8 and a 3′ untranslated region variant in TGFBR1 be related to decreased asthma risk. In a study on the Hispanic/Latino population in the United States, genetic predisposition to obesity was found as a risk factor for asthma, especially for childhood‐onset asthma in females.4
4 ENVIRONMENTAL RISK FACTORS FOR THE DEVELOPMENT OF ASTHMA
Many environmental factors can affect the risk of developing asthma. The American Academy of Allergy Asthma and Immunology (AAAAI) and European Academy of Allergy and Clinical Immunology (EAACI) have discussed emerging concepts and challenges in implementing the exposome paradigm and its application in allergic diseases and asthma. The complex network of exposome, genome, transcriptome, proteome, epigenome, and metabolome in driving the disease phenotype and endotype is described.20
Air quality has an influence on asthma symptoms and on triggering asthma attacks. Kim et al. correlated measurements of air pollutants around patients’ houses, including particulate matter (PM), with asthma status and with the frequency of innate immune cells (ILC) in induced sputum. A significant positive correlation between the amount of PM with a diameter ≤10 µm and asthma control was reported accompanied by an increased frequency of ILC2s in induced sputum.6
Recently, Yang et al. showed prenatal exposure to PM with an aerodynamic diameter of smaller than 10 μm (PM) has a higher association with airway hyper‐responsiveness (AHR) and the risk of a new diagnosis of asthma at early school age than current and lifetime exposure. Therefore, stricter monitoring and avoidance of exposure to PM might considerably reduce the onset of future asthma development in schoolchildren.21 Indoor air contaminants, containing endocrine‐disrupting chemicals (EDCs), can also increase the risk of asthma, as shown by Paciencia et al22 The authors assessed the association between EDCs exposure and asthma in children from 20 schools and found that increased individual and combined EDC levels were present in classrooms having more children with asthma and with an increased prevalence of nasal obstruction symptoms in the previous 3 months. EDCs are associated with changes in the autonomic nervous system, thus suggesting that EDCs may increase parasympathetic activity, resulting in a subsequent increase in the risk of asthma.22
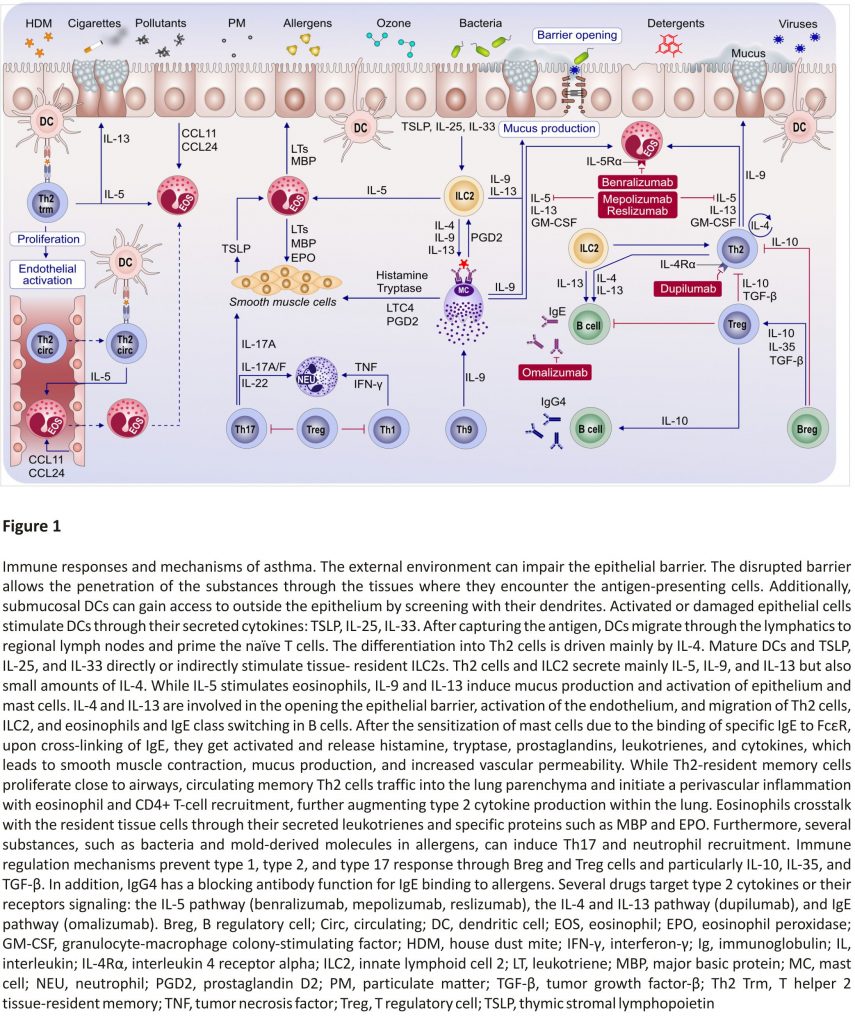
Pollutants can induce type 2 over type 1 response in the airways of an allergic asthma mouse model upon costimulation with the house dust mite (HDM) allergen as shown by Brandt et al (Figure 1).23,24 Increased IL‐33 signalling in the airway epithelium upon diesel exhaust particle (DEP) exposure can exhibit a synergistic effect with the type 2 cytokines IL‐5 and IL‐13 leading to severely increased AHR as well as a resistance to treatment with dexamethasone. IL‐5/IL‐13/IL‐17A coproducing CD4+ effector T cells in the lungs of DEP and allergen costimulated mice were identified as potential promoters of asthma exacerbations.
The activation of Aryl hydrocarbon receptor (AhR) exacerbating an allergic response was previously demonstrated. Weng et al25 showed that DEP activates AhR and with upregulation of IL‐33, IL‐25, and thymic stromal lymphopoietin (TSLP) by means of T helper cell (Th) 2 activation. This finding further supports the link between pollution and allergic severe asthma. Another recent study has reported AhR signalling critical role in the benzo(a) prene and Der f 1 co‐exposure, leading to epithelial cytokine release through regulation of reactive oxygen species (ROS) generation.26 Future studies on the AhR‐ROS axis may provide new therapeutic approaches to asthma.
Artemisia pollen allergy is a major cause of asthma in Northern China. Gao et al observed that the frequency of sensitization and the IgE levels related to the four main allergens (nArt v 1, nArt ar 2, nArt v 3, and nArt a 7) were significantly lower in subjects from the south of China compared to those from the north, who were more likely to have allergic asthma. The authors also reported that the co-sensitization to at least three of the most frequent allergens (Art v 1, Art v 3, and Art an 7) results in a higher risk of allergic asthma.27
The most common allergen in allergic asthma is group‐1 grass pollen. A wave of exacerbations of allergic asthma can be observed in fall when the grass has undergone senescence and turned to straw, where mould lives. Alternaria alternata spores from the surface of straw have been shown to carry the same allergens like grass pollen and probably inducing allergen‐mediated exacerbations in allergic asthma patients.28
Besides exposure to allergens, de novo sensitization to Aspergillus fumigatus is also becoming recognized as a risk factor for asthma patients. From baseline to follow‐up over a 10‐year observation period, asthmatic patients acquired noticeably increased frequencies for specific IgE levels to rAsp f 1.29 The presence of A. fumigatus was associated with reduced BAL macrophages, increased BAL levels of IL‐4, IL‐6, IL‐10, IL‐13, and TNF‐α, and increased plasma IL‐4, IL‐6, IL‐10, IL‐13, IL‐17, and TNF‐α. However, there was no relationship between the presence of A. fumigatus in the asthmatic airways and disease severity or control.30
Epidemic thunderstorm asthma is an emerging public health threat triggered by a combination of thunderstorms and massive loads of small pollen allergen particles, and people without a prior history of asthma can also be affected. D’amato et al5 investigated why thunderstorms are associated with a rapid increase in asthmatic patients in need of urgent medical care, due to asthma attacks. Thunderstorms can bring allergen particles down to ground level, and rainfall during thunderstorms is able to rupture pollen grains and release bio‐aerosols containing allergenic particles that penetrate deeply into the airways with acute severe allergic inflammation leading to asthma attacks with hospitalizations in people never experiencing before an asthma attack and suffering only of allergic rhinitis (AR) symptoms. A study from Melbourne investigated risk factors to predict severe asthma attacks requiring hospital admission during a thunderstorm. Odds for hospital admission were higher in Asian patients born locally compared to those born overseas. Non‐Asian patients had the lowest odds for the hospital admission. This suggests gene‐environment interactions playing a role in the susceptibility to severe thunderstorm asthma.31 Furthermore, Lol p 5, the major allergen of ryegrass pollen was reported to be responsible for triggering an epidemic of thunderstorm asthma.32 A systematic review and meta‐analysis on the possible link between pollen exposure and asthma hospital admissions in children and adolescents aged <18 years with emergency department attendance demonstrated ambient grass pollen as an important trigger for childhood asthma exacerbations requiring emergency department attendance, especially in relation to thunderstorm asthma.33
4.1 Smoking and e‐cigarettes
The association of asthma development and smoking has been extensively studied for conventional cigarettes.34 Extensive hazard to the respiratory system has been recently reported in multiple studies.35-38 A study evaluated the impact of electronic cigarettes and heated tobacco products on asthma and AR. The survey in Korean middle and high school students could not determine these tobacco products to be a risk factor on their own; however, in combination with conventional cigarettes, they could worsen asthma and AR status of children.39
4.2 Supplements, nutrients, and anti‐acid medications
In a recent review, Venter et al40 highlighted the potential roles of fatty acid metabolism in biological mechanisms, human epidemiology, and intervention studies. The impact of genetics and the microbial dysbiosis on fatty acid metabolism are discussed briefly. The authors suggest focusing on the choice of the formats (ie, food versus supplement) and standardized doses in clinical studies which will make easier to investigate their roles in the prevention and treatment of allergies and asthma. EAACI’s position paper recommends a diverse diet for the infants and consensus‐based definitions, which is a benefit for further studies.41 A study from Korea found that consumption of fast food was related to asthma incidence in adolescents but not in adults, whereas instant noodles had more impact in adults than in adolescents. No relation was found between asthma and the intake of vegetables and fruits, which might be confounded by the generally high intake of healthy food by Koreans as background nutrition.42
Another supplement, which was evaluated as an early prevention strategy for asthma, is vitamin D. Vitamin D supplementation remains a controversial issue, because of several recently published negative studies. Vitamin D supplementation of pregnant mothers did not reduce the incidence of asthma in children at 6 years of age. However, it might provide benefits by reducing the preschool wheezing episodes.43,44 A prospective study with maternal‐infant cohort showed that during the 2 and 5 years of observation, there is no association between vitamin D exposure antenatal or after birth and the progression of allergic disease.45 In addition, antenatal supplementation with vitamin D did not prevent the development of asthma or recurrent wheeze. Earlier results by the same group suggested that antenatal vitamin D lowers the risk of the offspring developing asthma by 3 years of age; however, these effects were lost by age 6.44
Tomita et al46 suggested a relationship between the use of acid‐suppressive medications, such as histamine 2 receptor antagonists and proton pump inhibitors, and the occurrence of adult‐onset asthma.
5 MICROBIAL DYSBIOSIS AND ASTHMA
Nasal, lung, and gut microbiota play several important roles in the development, regulation, and maintenance of healthy and asthmatic immune responses. Dysregulation of microbiota‐related immunological and metabolic processes impacts the onset of asthma, its clinical characteristics, and treatment response. 47,48 Antibiotic treatment and changes of the microbiome, especially early in life, are an important field of study since the microbiome has been associated with the health status of individuals in numerous diseases, including asthma.49 One‐year‐old children that had an immature microbiome were shown to have a higher risk to develop asthma later in life if their mother was asthmatic as well.50 A recent prospective study studied the connection between gut microbiota and the development of asthma in wheezing preschool children. No connection to the richness of microbes nor species diversity was found, but an increase in bacteria of the genera Escherichia and Gemminger at ages 2‐4 in children that were diagnosed as asthmatics, later on, was reported.51 Lee et al52 investigated the differences in the microbiome between young adults and elderly asthmatic and nonasthmatic individuals. They found upregulation in genes, such as relative abundances of microbiome genes associated with the pentose phosphate pathway, lipopolysaccharide biosynthesis, flagellar assembly, and bacterial chemotaxis, and nitric oxide production were higher in asthmatics than in nonasthmatics that could be related to increased inflammation and colonization of bacteria in young adult asthma patients. Furthermore, genes that could be related to the reduction of inflammation and degradation of air pollutants were higher in nonasthmatics of both age‐groups. In another study, the authors showed a reduction in prostaglandin E2 (PGE2) in the asthma group and an upregulation of the molecules that could be associated with airway inflammation such as arachidonic acid metabolites, lysine residues, and glycosaminoglycans.53 Together, these data suggest that alterations in the composition and function of the upper airway microbiome could influence asthma pathogenesis and that specific effects can be distinguished based on the age‐groups.
Probiotic bacteria interventions to prevent and to treat airway and allergic diseases are currently being evaluated. In a mouse model of asthma, Spacova et al54 used different strains of and intranasally administrations of Lactobacillus rhamnosus to observe the preventive effects of probiotics. Only L. rhamnosus GG strain treatment leads to a reduction in BAL eosinophil counts, lung IL‐5 and IL‐13 levels, and airway hyper‐reactivity. Thus, preventing the development of birch pollen‐induced allergic asthma by probiotics is strain‐ specific. Ingestion of bacterial lysate has also shown preventive effects on asthma. However, when extracts of Escherichia coli and Enterococcus faecalis were introduced into the diet of newborns, no significant effect on the occurrence of asthma, atopic dermatitis, AR, or sensitization to allergens was observed in 6‐ to 11‐year‐old children.55
Recently, the study of Michalovich et al56 identified additive effects of obesity and asthma in immunological responses and microbiota composition. The authors also showed that reduction in faecal Akkermansia muciniphila levels is associated with asthma severity. Their findings were confirmed in a mouse model in which administration of these bacteria reduces airway hyper‐reactivity and inflammation.56
Pulmonary microbial dysbiosis can influence the inflammation status of the host. The dysbiosis can be inherited, if the mother was treated with antibiotics during the pregnancy resulting in higher asthma rates in the offspring as shown by Alhasan et al57 Surprisingly, instead of the expected upregulation of type 2 cytokines in allergic offspring, a downregulation was found. Causalities are still unclear and will require specifically designed future studies to address this issue.
Microbial dysbiosis can also be induced by air pollution such as tobacco smoke, while other pollutants influence the epithelium more directly. Eguiluz‐Gracia et al58 reviewed the current understanding of indoor and outdoor air pollutants as well as the effects of climate change on human pulmonary health, highlighting how important clean air is for human health.
6 MOLECULAR MECHANISMS IN THE DEVELOPMENT OF ASTHMA
Immune system cells migrate to the lungs and display their functional properties to develop asthma.59 It was demonstrated that Th2‐resident memory T cells and circulating memory Th2 traffic into the lung parenchyma and initiate a perivascular inflammation to promote eosinophil and CD4+ T‐cell recruitment. Th2‐resident memory cells proliferate near airways and induce mucus metaplasia, AHR, and airway eosinophil activation. Transcriptional analysis revealed that Th2‐resident memory cells and circulating memory Th2 cells share a core Th2 gene signature, but also exhibit distinct transcriptional profiles (Figure 1).59
6.1 Molecular mechanisms in allergic and nonallergic asthma
Braga et al60 described the cellular landscape of airway lining at the single‐cell level. This comprehensive analysis in asthma identified dominance of TH2 cells interacting with structural and inflammatory cells. The presented data open a new perspective on lung biology and molecular mechanisms of asthma.60 A Th1/Th2 imbalance is commonly seen in allergic asthma, and it is shifted back toward Th1 by protein S. Protein S, an anticoagulant, anti‐inflammatory, and anti‐apoptotic glycoprotein, is associated with a reduction of AHR, lung tissue inflammatory cell infiltration, Th2 cytokines in the lung, and IgE levels. Asayama et al. showed that it could inhibit allergic asthma by upregulating the type 1 cytokines TNF‐α and IL‐12 while downregulating IL‐5+ Th2 cells.61 A downregulation of Th2 cells is also achieved by intraperitoneal injection of cysteamine, along with IL17+ Th17 and IL13+IL17+ Th2/Th17 cells, thus effectively inhibiting AHR in an allergic mouse model upon retreatment with the allergen.62 Lu et al reported high ILC2 levels in type 2 asthmatics, while non–type 2 asthmatics showed higher levels of Th17 cells and an inversed Th1/Th17 ratio.63 The pathways of Th2‐high and Th17‐high inflammation were reciprocally regulated.
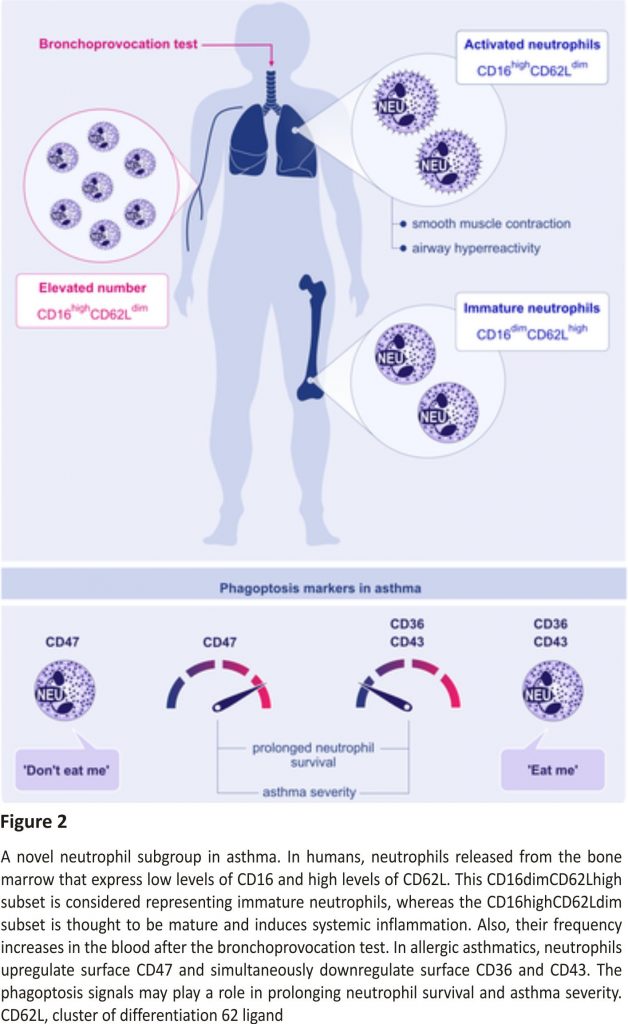
A neutrophilic inflammatory response or Th17 response is classified as non‐type 2 asthma. The potential mechanisms of non‐type 2 asthma have been well‐described in a comprehensive review article.64 Th17 cells are also shown to play a key role in allergic asthma by the secretion of inflammatory cytokines, including IL‐17A, IL‐17F, IL‐21, and IL‐23. Worth et al successfully demonstrated that genetic variants in IL‐17F, IL‐17A, and IL‐23 signalling genes (IL‐23A, IL‐23R, and IL‐12B) are associated with asthma. Their results also confirmed that IgE levels could influence the Th17‐related gene expression.65 Moreover, the IL‐17 levels and leukotriene (LT)B4 were shown to increase with disease severity in serum and sputum. Ro et al investigated the role of LTB4 receptors, BLT1 and BLT2, in a neutrophil‐dominant pulmonary inflammation murine model. BLT1 and BLT2 were proven as important in asthma development, and IL‐17 was identified as a key cytokine synthesized through the BLT1/2‐cascade.66 In humans, neutrophils released from the bone marrow express low levels of CD16 and high levels of CD62L. This CD16dimCD62Lhigh subset is considered representing the immature neutrophil, whereas the CD16highCD62Ldim subset is thought to be mature and induced systemic inflammation.67 Ekstedt et al recently found that this novel neutrophil subset, CD16highCD62Ldim, is increased in the blood following an inhaled allergen bronchial challenge bronchoprovocation test. They proposed that increasing neutrophil subgroups in allergic asthma might offer new opportunities in advancing allergic asthma research (Figure 2).67
“Eat me” and “Don’t eat me” signals are an integral part of phagoptosis, and thus, in neutrophils, they are important for the resolution of pulmonary inflammation as prolonged survival of airway neutrophils is directly related to asthma severity.68 In allergic donors, neutrophils showed an upregulation of CD47 (“don’t eat me”) and a simultaneous downregulation of CD36 and CD43 (“eat me”) compared to healthy controls (Figure 2).69 Additionally, less mRNA for CCL4 and CCL20 (significant for the resolution of a pulmonary inflammation) was found in airway neutrophils of allergic asthmatic donors than in healthy controls, which causes dampening in monocyte migration to the site of resolution.
One of the characteristics of the asthmatic lung is airway remodelling. Whether airway inflammation and remodelling in asthmatics can be related to persistent airflow obstruction was evaluated in a recent study that showed in asthmatics with persistent airflow obstruction increased airway smooth muscle area, decreased periostin and transforming growth factor‐beta (TGF‐β), and chymase‐positive cells compared to a patient with nonpersistent obstruction.70
A recent study has introduced an in vivo molecular platforms to elucidate both disease mechanisms and therapeutic targets of virus‐associated and non– virus‐associated asthma exacerbations. A group of exacerbation‐related modules included SMAD3 signalling, epidermal growth factor receptor signalling, extracellular matrix production, mucus hypersecretion, and eosinophil activation.71 Another study in infants suffering from rhinovirus bronchiolitis showed that concentrations of IL‐4, IL‐5, IL‐13, and TSLP were correlated with a higher risk of asthma onset in childhood.72 A study with 5‐year‐old children demonstrated that the development of atopic asthma in children with early rhinovirus‐induced wheezing was associated with differentially methylated genomic regions. The strongest methylation changes were observed in the SMAD3 gene promoter region at chr 15q22.33 and in introns of the DDO/METTL24 genes at 6q21.73
Respiratory syncytial virus (RSV) infection affects a large number of the population in the early years of their life and is associated with increased asthma risk. Schuler et al. show that uric acid and IL‐1β play a role in the mechanism, and their inhibition can prevent the development of asthma after neonatal RSV infection, thus being a possible therapeutic target.74 A prospective study suggested infants suffering from bronchiolitis at less than 6 months of age have a twice higher risk of doctor‐diagnosed asthma after follow‐up for 11‐13 years compared to general Finnish population and that the RSV was the main reason for bronchiolitis in these infants.75 The EAACI Influenza in Asthma Task Force performed a scoping review about the influenza burden, prevention, and treatment in asthma. In this review, vaccination conferred a degree of protection against influenza illness and asthma‐related morbidity to children with asthma, but not to adults with asthma. Although influenza vaccines appeared to be safe for asthmatic patients, there is a lack of data regarding efficacy in adults.76
Suojalehto et al examined the protein expressions from nasal brush samples from work‐related asthma patients and healthy controls using the proteomic approach. Work‐related asthma patients often are exposed to welding fumes and aerosols composed of hazardous metals and gases. The nasal brush samples are a relatively noninvasive specimen containing proteins secreted from epithelial and inflammatory cells. Their results indicated that the nasal epithelial proteome of the work-related asthma patients are highly enriched in processes related to inflammatory and calcium signalling, free radical scavenging and oxidative stress response, and metabolism.77
6.2 Molecular mechanisms in asthma due to obesity
Felix and Kuschnir78 pointed out that arginase inhibitors could act beneficially for obese asthma patients by upregulating the L‐arginine/asymmetric dimethylarginine ratio as an addition to a previous article by Meurs et al.79 The reply by Meurs et al80 agreed and suggested an even more direct link is pointing to the previously known increased arginase expression and activity in obese asthma patients. However, the mechanism behind this is still unknown, opening up avenues for future research.
6.3 Novel molecules and pathways
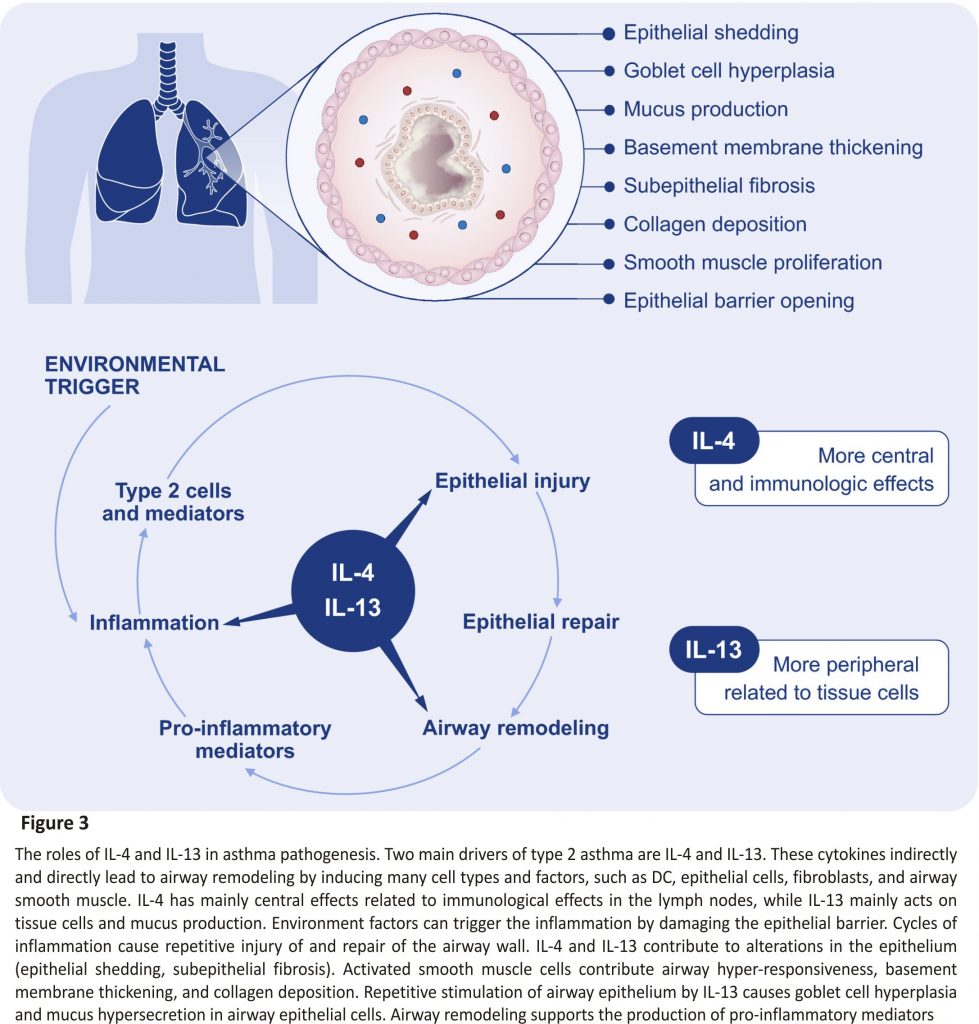
The type 2 immunity is mainly driven by IL‐4 and IL‐13 signalling which share the common receptor subunit interleukin‐4 receptor alpha (IL‐4Rα) (Figure 3). Withdrawal of IL‐4Rα signalling prevents the development of AHR, eosinophilia, and goblet cell metaplasia in allergen‐ sensitized mice. However, the IL‐4Rα‐ deficient mice do not develop type 17 immunity after allergic sensitization.81
The increased serum IL‐33 levels in asthma patients have been linked to the activation of mast cells. To characterize the mechanisms of IL‐33 contribution to asthma development, Ro et al82 used knockdown or pharmacological inhibitors in bone marrow-derived mast cells and animal model. The study revealed that the “MyD88‐5‐/12‐LO‐ BLT2‐NF‐κB” cascade contributes to the IL‐33 signalling to induce IL‐13 synthesis in mast cells, which may represent a new therapeutic target for severe asthma.82
Lv et al investigated the role and the mechanisms of IL‐37 in type 2‐mediated allergic lung inflammation in HDM‐induced murine asthma model. They found IL‐37 impairs HDM‐induced asthma, most likely by preventing IL‐4/IL‐13‐induced chemokine ligand (CCL) 11 production from fibroblasts and airway smooth muscle cells through its direct effect on tracheobronchial epithelial cells.83
In a murine allergic asthma model, IL‐4 receptor‐α blockade decreased serum IgE and IL‐5 levels and increased the level of IgG1, IgG2a, IgG2b, and IgG3 prior to/during sensitization. Thus, following the IL‐4 receptor‐α blockage, an immediate immunoglobulin response is induced accompanying the suppression of type 2 cytokines with a potential long‐lasting reduction in Th2‐biased T regulatory (Treg) cells.84
Chitotriosidase (chitinase 1, Chit1) has been known as regulator and stimulator in Th2 responses. Hong et al studied the possible mechanisms and their role in the pathogenesis of allergic asthma.85 Significantly elevated levels of Chit1 in the sputum of patients with childhood asthma were reported. Moreover, in the absence of Chit1 molecule, forkhead box P3 (Foxp3)+ Treg cell frequency decreased in the lungs of mice besides TGF‐β1 levels, which suggests a protective role in asthmatic airway responses by regulating TGF‐β secretion and Foxp3+ Treg cells.
A study performed by Guan et al86 have reported that reduced monocytic myeloid‐derived suppressor cells (M‐MDSC) may result in abnormal T responses with the increase in Th2 and Th17 cells and decrease in Treg cells in asthma patients. These results suggest a new immune regulatory mechanism in the pathogenesis of asthma open for further research.86 To define whether there is a deficiency in Breg subsets, Wirz et al87 compared the percentages of IL‐10‐producing Breg subsets in peripheral blood from patients with asthma and AR. They demonstrated that there is no difference in numbers of Bregs in the patients when compared to healthy controls.87
Current research suggests that several receptors are involved in asthma pathophysiology, including PGD2 receptor 2 (DP2 or CRTH2), as well as colony-stimulating factor 1 receptor (CSF1R). The DP2 receptor is an essential regulator in allergic asthma because it can be activated by both allergic and nonallergic stimuli.88 The activation of the DP2 receptor pathway increases both the airway smooth muscle mass and vascularization in airway walls, resulting in downstream effects on asthma development. Interestingly, airway epithelial cells secrete CSF1 into the alveolar space in response to aeroallergen. Moon et al. demonstrated that inhibition of the CSF1‐CSF1R signalling pathway could suppress sensitization to aeroallergens and subsequent allergic lung inflammation in mice with chronic asthma. They conclude that inhibition of CSF1R is a potential new target for the medical treatment of allergic asthma.89
Kim et al90 showed that the ceramide/sphingosine‐1‐phosphate ratio can discriminate between two different asthma endotypes. Sphingosine‐ 1‐phosphate (S1P) was found to positively correlate with the percentage of platelet‐ adherent eosinophils, indicating eosinophilic inflammation. The percentage of CD66+‐activated neutrophils positively correlates with C16:0 ceramide levels, indicating neutrophilic inflammation. An upregulation of ceramide‐mediated pro‐apoptotic signals was found in patients with higher CD66+ neutrophils. For both eosinophilic and neutrophilic pathways, genetic SNPs were found. Increased ceramide levels may also contribute to the development of obese asthma. Choi et al91 found multiple ceramides (C16:0, C18:0, and C20:0) to accumulate in obese mice as a result of a high‐fat diet, inducing AHR and inflammation. Increased expression of ceramide synthase (CerS) 1 and CerS6 was found in the lungs, and CerS6 was identified as a potential future therapeutic target for obese asthma.
Asaduzzaman et al92 studied cockroach‐induced chronic murine asthma models by using a specific inhibitor of proteinase‐activated receptor‐2 (PAR2) to identify the role of PAR2 signalling on AHR and airway inflammation /remodelling. They showed that administration of an anti‐PAR2 antibody significantly inhibited AHR, inflammation, and collagen accumulation in the lung tissue. The authors suggest that PAR2 blockade may be a successful therapeutic strategy for human allergic airway diseases.92
A few studies have been performed to reveal the effects of medical treatments with ILCs in allergic airway diseases so far. The mechanisms in action of glucocorticoid therapy on ILCs were assessed in a prospective study by Yu et al93 Their data showed the administration of glucocorticoids regulates ILC2s via MEK/JAK‐STAT signalization pathways in asthma patients.
To test the role of MUC1 membrane mucin in uncontrolled severe asthma, the recent study analyzed the association of MUC1‐CT (cytoplasmic tail in the C‐terminal subunit) with corticosteroid efficacy in vitro and in vivo models. The results suggested that MUC1‐CT has an important role in the modulation of the anti‐inflammatory effects of corticosteroids and may be a promising new approach for the treatment of asthma.94
MicroRNAs (miRNAs) are secreted in extracellular vesicles and regulate signalling pathways by being transferred between cells. Recently, Bartel et al characterized the miRNA secretion in extracellular vesicles from normal bronchial epithelial cells treated with IL‐13 to induce an asthma‐like epithelium. They observed that miR‐34a, miR‐92b, and miR‐210 were involved in the early development of a Th2 response in the airways and asthma.95
Uwadiae et al96 studied the role of T follicular helper (TFH) cells of allergen airway disease in a mouse model of allergic asthma. They found that TFH cells accumulate beside the germinal centre B cells with constant allergen exposure. Furthermore, blocking the inducible costimulatory (ICOS) signalling disrupted the TFH cell response; however, it did not have an impact on the differentiating of other CD4+ T‐cell subsets. Based on these observations, the authors suggest that TFH cells have critical roles in the regulation and the ICOS/ICOS‐L pathway can be a novel therapeutic target in allergic airway disease.96
7 COMORBIDITIES OF ASTHMA
Most research of treatments for airways diseases has been restricted to patients who meet standard definitions of either chronic obstructive pulmonary disease (COPD) or asthma, yet to distinguish COPD from asthma in adult patients who have clinical features of both can be challenging.97 Asthma‐COPD overlap syndrome (ACOS) is a poorly described condition, which shows a clinically different approach compared to asthma or COPD alone. Wang et al estimated that among newly diagnosed adult-onset asthma patients in Southern Finland, the prevalence of ACOS is 6.6%. Most of the cases with ACOS were older, with lower education, more often men and current or former smokers, when compared to the asthma‐only cases. Interestingly, having additionally other respiratory diseases or allergic diseases was less common among the ACOS cases than among asthma‐ only cases, but familial asthma was associated with an increased risk of ACOS.98
Asthma can come together with the local AR, because of united airways. These patients show seasonal or perennial asthma‐like symptoms, negative skin prick test, and/or specific IgE, but display positive bronchial and/or nasal allergen provocation test responses triggered by HDM. These features suggest a new phenotype of asthma by the absence of systemic atopy, namely local allergic asthma.99
Sanna et al reported that the incidence of co‐existing AR, allergic dermatitis, and allergic conjunctivitis, was positively correlated with the risk of adult‐onset asthma, and this tendency was decreased with the increasing of age.100 In a recent review, Samitas et al101 discussed the one airway concept which suggests disease mechanisms that take place in the upper airway might be reflected by lower events. Additionally, bronchial fibroblasts also have been known for their role in airway remodelling by regulating epithelial cell functions.
Sharma et al102 reviewed experimental and clinical information for specific mediators of asthma and obesity alone or comorbidities, such as adipokines, cytokines, and fatty acids which involve in the polarization of macrophages, particularly. M1 and M2 macrophages might co‐present in distinct organs and may impact asthma and obesity differently. M1 macrophages are suggested beneficial for asthma, while M2 macrophages contribute to the pathology of both asthma and obesity. Potential therapeutics for obese asthmatics by breaking the macrophage‐mediated cycle between obesity and asthma were suggested. Recently, Chen et al indicated that early pubertal maturation is related to childhood‐onset asthma in both genders.103 Besides, obesity together with early pubertal maturation can increase the risk of asthma, which implies the importance of the body mass index (BMI) control to prevent early pubertal maturation and asthma onset. Using 26 BMI‐associated SNPs, Skaaby et al. evaluated the causal effect of BMI on asthma, hay fever, allergic sensitization, total serum IgE, forced expiratory volume in one second (FEV1), and forced vital capacity. As a result of genetic analysis of 162 124 participants, the authors concluded that increasing BMI is causally related to a higher prevalence of asthma and decreased lung function, but not with hay fever or biomarkers of allergy.104
Future directions in asthma therapy, compromise both the optimization of known treatments and the implementation of novel approaches. As an example of the first option, Tay et al highlighted the importance of comorbid “treatable traits” in difficult asthma, where they concluded that treatment of most extra‐pulmonary comorbidities (eg, rhinitis, chronic rhinosinusitis, gastroesophageal reflux, or obesity) improves asthma outcomes. They suggested a targeted assessment of patients suffering from difficult‐to‐treat asthma, including questionnaires, specialist assessments, and tailored referrals, preferably before initiating phenotype‐driven biological treatment.105 However, it is noteworthy that some common asthma comorbidities (eg, chronic rhinosinusitis) might profit from the “biologicals approach” as well.106
An important, yet often neglected aspect of asthma is the high prevalence of anxiety and depression in this patient group. A recent study reported on the mental health of asthma patients and found a positive correlation between the severity of anxiety and depression with less well‐controlled asthma symptoms.107
Generally, the analysis of comorbidities in asthma patients is extremely relevant, as shown by a study by Lemonnier et al. The authors have identified gene expression patterns in peripheral blood mononuclear cells that are associated with the combined presence of asthma, AR, and/or dermatitis in the mechanisms of the development of allergy and epigenetic variation and childhood asthma in Puerto Ricans cohorts. Eight common genes were overexpressed in multi‐ morbidities, while no genes were observed to be differentially expressed specifically to the presence of asthma.108
In another study in adolescents and adults with asthma and rhinitis (A+R+), multimorbidity evaluating the IgE polysensitization toward various aeroallergen components showed frequent IgE sensitization to pollen and indoor allergens in A+R+ and contrasting with pollen allergens in rhinitis alone.109
Comorbidities also impact mortality in asthma. In a 15‐year follow‐up cohort of adults with asthma, the development of COPD, malignant respiratory tract neoplasms, and cardiovascular diseases were the main reasons for increased mortality. AR and/or allergic conjunctivitis were associated with decreased mortality but with increased morbidity.110
8 ASTHMA DIAGNOSIS, PRECISION MEDICINE, AND BIOMARKERS
Asthma should be correctly diagnosed as early in life as possible according to the latest clinical guidelines. Branco et al reported that 1.3% of children were previously undiagnosed asthmatics, providing evidence of underdiagnosed asthma in both pre-and primary school children in both urban and rural areas.10
Postma et al. reported the baseline data from the multi-centre, international study specifically designed to explore the relevance and extent of small airway dysfunction (SAD) in asthma. In the largest such study to date, the team developed a clinical SAD score and showed that SAD is present across all asthma severities, but consistently more so in severe asthma. The clinical impact of SAD in asthma is further explored in the longitudinal study.111 A study proposed the addition of oscillometry together with spirometry as it proved useful to assess earlier changes in responders to treatment with benralizumab.112
Dunn et al113 evaluated asthma in the elderly and late‐onset asthma in adults. Elderly asthmatics have higher rates of morbidity and mortality compared to younger patients. It is more likely that the disease is undiagnosed and undertreated. Elderly patients more often have a non–type 2 asthma endotype, with a Th17‐mediated pathology, which is also less responsive to traditional therapies such as ICS. Therefore, it is important that more elderly patients are included in clinical trials.
Nonsteroidal anti‐inflammatory drug (NSAID)‐exacerbated respiratory disease (NERD) is a severe eosinophilic asthma phenotype.114 It has been well defined as an inflammatory phenotype responsive to corticosteroids. Although no absolute/consistent cutoff values have been established, subanalyses show an overall better response in patients with more inflammation, defined by higher blood eosinophil levels.115 Recently, Celejewska‐Wójcik et al demonstrated that three distinct NERD subphenotypes reflect differences in inflammatory response measured by airway eosinophils and the ratio of logLTE4/logPGE2 in induced sputum. 116 Mastalerz et al117 studied airways PGE2 in induced sputum and reported that NERD subjects had higher levels of PGE2 before aspirin challenge compared to controls. After exposure to aspirin, PGE2 levels significantly dropped, which was not the case for aspirin‐tolerant asthmatic individuals. The inhibition of bronchial PGE2 biosynthesis can trigger bronchoconstriction in NERD. Chen et al118 showed that the levels of exhaled PGE2, LTB4, LXA4, and LTE4 may efficiently differentiate asthmatic children from healthy controls. Especially, the measurement of LTB4 and lipoxin A4 together with FeNO and FEV1 might help for the better diagnosis of asthma.
A nationwide Japanese prospective study has investigated 190 patients with near‐fatal asthma exacerbation. By analyzing asthma symptoms over the 2‐week period before their admission, the authors could define three different clusters of symptoms. Analysis of clusters indicated the most relevant factors to be assigned to three different clusters, such as a degree of ICS or ICS/ LABA compliance, low or high perception of dyspnea, and hypersensitivity to environmental stimuli.119
Additional to clinical trials, the analysis of real‐world data is very important to confirm effectiveness also in larger, more diverse patient groups. Jutel et al analyzed changes in AR progression and asthma status after HDM allergen immunotherapy (AIT) based on data on prescription medicine consumption. They found that treatment of AR patients with HDM allergoid can ameliorate asthma symptoms, slow down asthma progression, and reduce the general incidence of asthma as compared to untreated control groups.120
Asthma‐inducing agents are also present at many workplaces. Occupational asthma is often hard to diagnose since the reference test (specific inhalation challenge) can only be conducted in a few centres worldwide. Recently, a model to improve diagnosis of occupational asthma without specific inhalation challenge was published on the basis of a Canadian dataset and was successfully validated in a European population.121 Clinicians could use this model with decision making on referral to a specialized centre in the future. Van der Plas et al122 studied the differences between sensitization to high-molecular-weight proteins and low-molecular-weight chemicals in occupational asthma. Asthma caused by high-molecular-weight chemicals showed a stronger association with rhinitis, conjunctivitis, atopy, and early asthmatic reactions and had a higher risk of airflow limitation, while low‐molecular‐weight agents were associated with chest tightness, late asthmatic reactions, and increased risk of severe exacerbations. Beretta et al highlighted that the measurement of nonspecific bronchial hyper‐responsiveness alone is often not enough to diagnose occupational asthma. Combining it with the assessment of FeNO levels and sputum eosinophil count significantly increases the sensitivity and accuracy of the method, improving the identification of subjects, who may have occupational asthma and therefore require further testing.123
Multiple omics, big data, and systems biology have demonstrated a profound complexity and dynamic variability in asthma between individuals, as well as between regions. Reliable diagnosis of asthma and the monitoring of its severity are challenging particularly in daily clinics. Asthma is an umbrella term, including several distinct phenotypes and endotypes, which are characterized by specific cellular and molecular immune response patterns.47,115,124,125 The main asthma endotypes, type 2 and non–type 2 inflammation, are broader described in the section below. Type 2 allergic asthma is defined by IgE, IL‐13, IL‐4, IL‐5, and eosinophil responses and covers more than 50% of asthma endotypes.115
Boudier et al. highlighted the value of unsupervised asthma phenotypes research, including multiple asthma characteristics, for understanding the long‐term evolution of asthma patients. Using a cluster‐based model developed for longitudinal data, asthma phenotypes were identified in a large population of adults with asthma 20 years after recruitment.126 This model was developed by taking into account two-time points and nine variables combining clinical and functional characteristics, such as respiratory symptoms, asthma treatment, allergic characteristics, lung function, and bronchial hyper‐responsiveness. These cluster‐based asthma phenotypes showed a stronger long‐term clinical prognosis compared to phenotypes classically used in epidemiological studies, allowing a strong tracking of lung function over the life course to better tailor asthma management strategies.126
Ivanova et al reviewed the role of “omics” technologies in asthma.127 Although omics data studies have several limitations, usually, due to the limited sample size and the complexity of the data and all its interactions, different insights were acquired. Another review also highlighted the importance of omics data for molecular phenotyping, defining the endotypes, and identifying pathways and mechanisms, such as type 2‐high and type 2‐low.1 In regard to this purpose, the transcriptome and protein levels in three different mouse models for eosinophilic, mixed, and neutrophilic asthma were analyzed. The authors found that differential expression of tight junctions, mucin, and inflammasome‐related molecules in distinct inflammatory phenotypes of asthma may be linked to the pathophysiology and might reflect the differences observed in the clinic.125 Eosinophil and neutrophil dominant phenotypes were described in children with asthma as well. The neutrophil dominant phenotype was associated with the biggest differences compared to the other asthma phenotypes. The vast majority of the differentially expressed genes was associated with corticosteroid response, and the neutrophilic phenotype was associated with corticosteroid nonresponsiveness.128
Severe asthma is a heterogeneous disorder, including different clinical characteristics (phenotypes) and immunopathological pathways (endotypes). The identification of noninvasive biomarkers that are able to predict treatment response and assist in designing personalized therapies for severe asthma patients is demanding.
Eguiluz‐Gracia et al broadly reviewed recent developments in biomarkers in allergic diseases, highlighting the importance of eosinophils in allergic asthma diagnosis and management.124
According to an EAACI position paper in 2019, biomarkers for the clinical and inflammatory phenotype of asthma were summarized as follows (1) type 2 asthma: (a) serum IgE, (b) blood and sputum eosinophils, and (c) FeNO; (2) non‐type 2 asthma: (a) sputum neutrophils and (b) blood and sputum eosinophils.115 However, the etiology of asthma with non‐type 2 inflammation is less clear.
Eosinophils as biomarkers: Sputum eosinophilia is the most useful biomarker in asthma. In general, sputum eosinophilia is associated with steroid responsiveness. Although there is no standardized cutoff, a blood eosinophil count of 300 cells/μL and the normal range for sputum eosinophilia defined as 1–2% have commonly been used as a threshold to indicate eosinophilic asthma.115 Higher blood or sputum eosinophil count has been assessed to be a sensitive and practical predictive biomarker for biological therapies targeting allergic and/or eosinophilic pathways in patients with severe asthma.129-131 Systematic reviews showed the efficacy and safety of benralizumab, dupilumab, mepolizumab, omalizumab, and reslizumab for severe eosinophilic asthma and allergic asthma.129,130 A high blood eosinophil count (>300 cells/μL) has been reported as a potential biomarker to predict successful treatment effects of omalizumab in children with severe allergic asthma.115 Sputum eosinophilia also adequately predicts response to biologics. Patients with refractory asthma are more likely to respond to anti‐IL‐5 or anti‐IL‐4/IL‐13‐ targeted treatment if they have sputum eosinophils of >3% or ≥ 300 cells/μL blood eosinophils.115, 129-131
For evaluating treatment success with mepolizumab and patient stratification, possible biomarkers were investigated in a post hoc study of phase III clinical trial data. The results of this study reinforce the use of peripheral blood eosinophil counts and eosinophil‐derived neurotoxin as predictive biomarkers.132
The transcriptomic data from bronchial biopsies of European U‐BIOPRED cohort patients showed that MMP‐10 and MET genes were significantly overexpressed in severe asthma. These results demonstrated that MMP‐10 and MET play an important role in pathways of airway remodelling and cellular inflammation that are associated with submucosal eosinophilia.133
Recent studies have shown that eosinophils can also display protective regulatory properties in asthma. In a recent study, Pineros et al134 provided ex vivo and in vivo evidence that mouse and human eosinophils are capable of rapid capture and inactivation of respiratory viruses. They also showed that eosinophils from asthma patients displayed a reduced capacity to bind the virus, which may lead to less effective virus inactivation.134 These results underlie the in vivo antiviral activity of eosinophils and the pathogenesis of virus‐induced asthma exacerbations. Another study conducted by Tarancon et al. evaluated eosinophils during Mycobacterium tuberculosis infection in an experimental model. They observed that eosinophil production in the bone marrow is weakened in Mycobacterium tuberculosis infection and protects against asthma.135
The role of eosinophils in personalized asthma treatment remains controversial. In a real‐life study, Bagnasco et al showed no correlation between peripheral blood eosinophils count with the clinical, functional, and biological outcome changes in asthma patients.136, 137
Not only frequencies of eosinophils can serve as a biomarker of asthma severity 124 and response to the treatment.138 Rodrigo‐Muñoz et al139 recently reported a set of 14 miRNAs stratifying eosinophils from asthmatic patients and controls. Interestingly, 3 of those miRNAs (miR‐144‐5p, miR‐185‐5p, and miR‐320a) were validated as asthma biomarkers in the serum, distinguishing not only asthma status but also the severity.139
Other immune cells as biomarkers: The level of human circulating neutrophil extracellular trap but not eosinophil extracellular trap can act as a potential marker for asthma severity and poor control.140 Another candidate marker for asthma severity could be the frequencies of circulating chemokine receptor (CCR) 10+ ILC2s and plasma CCL27 level. Conversely, CCR10+ ILC2s resemble the characteristics of ILC1s and display higher levels of T‐bet expression and increased production of interferon (IFN)‐γ compare to the CCR10‐ILC2 counterpart, which favours the controlling of allergic inflammation in asthma.141
IgE as a biomarker: IgE exerts several biological functions as an Fc receptor-bound antigen sensor for mast cells, basophils, dendritic cells (Dcs), T and B cells, and other cells in the allergic inflammation. Total serum IgE and allergen‐specific IgE have been strongly associated with asthma.115 Omalizumab is the recombinant humanized mAb that binds to the Fc region of IgE. Correlations between treatment response and baseline total serum IgE or antigen‐specific IgE levels are not clear, but serum IgE is used to dose omalizumab.115, 129, 130
8.1 Proteins as biomarkers:
Hoang et al showed that seed storage proteins (Ara h 1, 2, 3, 6), uteroglobin from the cat (Fel d 1), and lipocalin from the dog (Can f 1) demonstrated the strongest linkage to clinical markers of asthma severity. These results suggest their potential contribution as biomarkers in preschool asthma.142
A study in the Spanish population identified serum biomarkers for allergic or nonallergic asthma by using isobaric tags for relative and absolute quantitation (iTRAQ)‐based proteomics. It indicates that increasing levels of plasma insulin‐like growth factor‐binding protein acid‐labile subunit, which has a role in the regulation of insulin‐like growth factor pathway, could act as a potential biomarker for defining the severity of allergic asthma, whereas the proteins of the complement ficolin‐2 and mannan‐binding lectin serine peptidase 1 levels seem increased in nonallergic individuals.143
Exhaled Biomarkers: Measurement of biomarkers in exhaled breath (ie, FeNO) and exhaled breath condensate (ie, volatile organic compounds) are non-invasive and safe. FeNO is a widely accepted biomarker for type 2‐driven airway inflammation. Recently, a new subgroup of patients with high FeNO levels (>25 ppb) and low blood eosinophils (<300 cells/μL) was described. These patients showed a significantly higher number of sensitizations against aeroallergens compared to patients with low FeNO subgroups.115 FeNO is also associated with an increased risk of asthma exacerbations and a beneficial effect of ICS. Pavord et al investigated whether these biomarkers have prognostic value or predict the effects of regular or as‐needed ICS on exacerbations in patients with mild asthma.144 The open‐label, randomized controlled trial showed that the effects of as‐needed budesonide‐formoterol are independent of biomarker profile.144 Dupilumab improves asthma control, quality of life, and FEV1. The use of rescue medication is reduced above the minimally important clinical difference threshold only in patients with high blood eosinophils and high FeNO (blood eosinophils >300/μL and/or FeNO >50 ppb).131
The non‐T2 endotype covers both patients with a neutrophilic and a pauci‐granulocytic airway inflammatory pattern. Childhood asthma comprises more different phenotypes with a complex pathophysiology. Su et al. showed that neutrophil‐predominant asthma is the most severe asthma phenotype in children with a poor corticosteroid response.128 Less is known about biomarkers for pauci‐granulocytic asthma. The role of biomarkers in the non‐type 2 endotype has yet to be fully elucidated.
AIT is an allergen tolerance‐ inducing treatment for allergic diseases. There are no biomarkers that sufficiently predict response to AIT. The Allergen Immunotherapy User’s Guide summarized the potential biomarkers for monitorization of the clinical efficacy of AIT as follows: (a) IgE (total IgE, specific IgE (sIgE)/total IgE ratio), (b) subclasses of IgG (allergen-specific IgG, IgG1 and sIgG4, sIgE/IgG4 ratio), (c) IgE serum inhibitory activity for IgE (IgEFAB), (d) basophil activation, (e) chemokines and cytokines, (f) cell markers such as Tregs, Bregs, and DCs, and (g) in vivo biomarkers including provocation tests.145, 146
Digital asthma biomarkers: Exhaled breath analysis using an electronic nose (eNose) is a new technique. This tool has the potential to assess asthma control and tailoring asthma treatment.147, 148 In a study including participants between 6 and 18 years of age, Cavaleiro Rufo et al showed that the exhaled breath condensate volatilome analysis by an eNose has good accuracy for asthma identification being able to distinguish individuals with diagnosed pediatric asthma from those without the disease.149 Farraia et al demonstrated that the analysis of the exhaled volatile organic compounds profiles using an eNose could be used as a fast and non-invasive complementary assessment tool for the detection of uncontrolled asthma.147 Moreover, the eNose was able to identify individuals with persistent asthma under prescribed corticosteroid therapy, supporting the diagnostic ability of this method to identify individuals in need of corticosteroid therapy.149
Mobile Airways Sentinel Network [MASK] is an information technology-based tool that developed through ARIA studies, which can inform patient decisions based on a self‐care plan proposed by the healthcare providers and can increase self‐medication and share decision making in rhinitis and asthma multimorbidity.150 Using the MASK‐air app, 14 189 users and 205 904 days, a visual analog scale (VAS) days, have been recorded. VAS work correlates with other outcomes (VAS global, nose, eye, and asthma) but less well with a symptom‐medication score. VAS profile has potential for prevention, for the assessment of AR severity and progression, and for monitoring the drug effects in patients.151 Another study has determined the importance of mobile technologies in rhinitis control by using AR and its impact on asthma (ARIA) score.152 Subsequent to this study, Bousquet et al examined the use of mobile technology to get information in the change management of AR and asthma multimorbidity, with the aim of providing an active and healthy lifestyle for these patients.150 The European Innovation Partnership on Active and Healthy Ageing transferred innovation from the “Allergy Diary” to 22 reference sites or regions across Europe, aiming to compare the phenotypic characteristics of rhinitis and asthma multimorbidity in adults and the elderly, to assess the percentage of accepting the Allergy Diary in adults and elderly, and to understand the phenotypic characteristics and follow‐up treatment over 1 year of rhinitis and asthma multimorbidity.153
9 NOVEL TREATMENTS DRIVEN BY ASTHMA ENDOTYPES
Precision medicine, which is fitting very well asthma’s heterogeneity and complex pathogenesis, is becoming an overarching medical discipline that requires a good understanding of biomarkers, phenotypes, endotypes, genotypes, regiotypes, and theratypes of diseases.2 ICS have been the foundation for asthma treatment; however, inhaled or systemic corticosteroids can be ineffective in many patients with asthma. Few treatment options exist for patients with steroid‐resistant asthma. The recent development of a new class of biological agents that target airway type 2 inflammation has provided an opportunity for treating patients with corticosteroid refractory asthma (Figure 1).154
A significant amount of research supports the separation into type 2 and non–type 2 asthma endotypes.8,155, 156 Type 2 asthma usually has high sputum and blood eosinophil counts and high FeNO levels. The diagnosis of non–type 2 patients, who are usually unresponsive to the ICS treatment, remains a challenge. Some studies have suggested that elevated levels of circulating interleukin (IL)‐17, IL‐6, IL‐23, or other factors, such as bacterial infection and obesity are all involved in the pathogenesis of the non-type 2 asthma.157 Neutrophilia observed in these non‐type 2 patients was related to recurrent respiratory tract infection facilitated probably by a deficiency of local airway immunoglobulins. Asthma patients who received intravenous immunoglobulin exhibited higher levels of serum IgA and fewer infection exacerbations 12 months afterwards.158
Several earlier studies have highlighted the impact of high‐altitude treatment in atopic dermatitis and asthma.159, 160 A recent study by Boonpiyathad et al161 described the clinical and immunological changes after high‐altitude treatment in asthma patient subgroups and showed that clinical improvement is dependent on the asthma phenotype. Furthermore, high‐altitude treatment reduced the type 2 immune response and corrected the elevated CRTH2 expression and its dysregulated functions.161
Severe asthma is defined by the ERS/ATS criteria as either asthma requiring escalation to step 5 medical therapy (=high‐dose ICS in combination with a second controller and/or additional systemic corticosteroid therapy) to maintain asthma control or asthma that remains uncontrolled despite step 5 therapy.162,163 Only 5%‐10% of asthma patients fulfil these criteria, but they are responsible for >80% of the total asthma healthcare costs. Adverse effects due to an overload of corticosteroid therapy (resulting from an often combined nasal, cutaneous, and/or inhaled therapy) are recognized as a major contributor to the immense healthcare costs.162 Voorham et al showed in a matched, historical cohort study that patients undergoing long‐term systemic corticosteroid treatment suffered annually increasing healthcare costs compared to patients who do not receive systemic.164
Targeted therapies play a critical role in severe and difficult‐to‐treat asthma in adults, including monoclonal antibodies against IgE, blockage of IL‐4 and IL‐13 signalling, and anti‐IL‐5 and anti‐IL‐5 receptor therapies.165,166 This is partly why optimization of severe asthma therapy (ie, by reducing steroid use) received considerable attention in the past decades, with targeted approaches (eg, biologicals) (Figure 1).162 Currently, there are five biologicals approved for difficult‐to‐control asthma, targeting IgE (omalizumab), IL‐5 (mepolizumab and reslizumab), IL‐5Rα (benralizumab), and IL‐4Rα (dupilumab). These drugs were shown to have steroid‐sparing effects and reduce asthma exacerbations, as well as hospital admissions, in randomized control trials.106 Biological treatment of severe asthma also comes with a high cost for the healthcare system (it exceeds the recommended maximal cost per quality‐adjusted life‐year by far); thus, the selection of the patient is required to be rigorous.106,162 One difficulty in patient selection is due to the overlapping severe asthma phenotypes, such as severe allergic asthma and severe eosinophilic asthma. A multi-centre, open-label, single‐arm study conducted by Chapman et al. showed that a direct switch from omalizumab to mepolizumab (within 2‐4 weeks of the last biologic dose) is possible without any tolerability issues and can be highly beneficial for patients with severe eosinophilic asthma not optimally controlled by omalizumab.167,168 As crucial as the patient selection is for the initiation of biological treatment, the decision in which patients to discontinue it after long‐term usage is equally important. In a real‐life study involving children with severe allergic asthma, Deschildre et al demonstrated that omalizumab discontinuation could be safely proposed after at least 24 months of treatment in children with prolonged controlled asthma and no severe exacerbations for at least one year. They also showed that some phenotypic markers (female gender, allergic multimorbidity, and decreased lung function) should be taken into account, consistent with the findings from the persistency of response after long‐term therapy (XPORT) trial assessing omalizumab discontinuation effects in an adult population.169
The identification of treatment responders is of great significance for patient selection; however, validated biomarkers are currently scarce. For benralizumab, Mathur et al found several indicators of enhanced response of the drug in two phases III trials in patients with elevated blood eosinophil counts, decreased lung function, long‐term oral corticosteroid use, and nasal polyposis.170 In a small prospective study, Antonicelli et al. showed that forced oscillation technique (a non-invasive method that provides information of the degree of obstruction of the respiratory system) could detect specific mepolizumab‐induced changes in peripheral airway function in patients undergoing treatment for severe eosinophilic asthma.171
EAACI recently launched its guidelines for the use of biologicals in severe asthma. Recommendations follow the GRADE approach for each biological and each outcome. In addition, a management algorithm for the use of biologicals in the clinic is proposed, together with future approaches and research priorities.172
Fokkens et al stated that it is likely that biologics will become an alternative for sinus surgery in chronic rhinosinusitis with nasal polyposis, which is a frequent comorbidity of severe asthma.106
Optimizing patient empowerment and satisfaction by allowing self‐injection of currently approved biologicals administered subcutaneously is another approach.173 Another key future development could be the optimization of airway delivery of biological agents by using nebulized monoclonal antibodies.163
Another promising new approach is the use of arginase inhibitors which lead to an increase in nitric oxide levels, thus promoting bronchodilation and inhibiting airway inflammation. Currently, drugs based on arginase inhibition are under development.79 Other possible future targets in personalized asthma therapy include the epithelial cell-derived cytokines (eg, IL‐33 or TSLP), kinases (eg, JAK and Pi3K), and the PGD2 (acts as a pro‐inflammatory mediator) (Figure 1).162,163 A comprehensive review by an EAACI task force174 highlighted the complex roles of eicosanoids in asthma and allergy.
Type 2 inflammation‐independent asthma represents a critical unmet medical need in the search for potential drug targets, as such patients show a decreased response to asthma therapies targeting type 2 cytokines. By generating a noncompetitive inhibitory antitryptase antibody in humanized mouse and cynomolgus monkey models, Maun et al. provided the scientific rationale for clinical testing of such antibodies.175
Kere et al examined the association between biologicals, corticosteroids, and DNA methylation in peripheral blood cells from asthmatic children and found no evidence that ICS or other asthma medications affect peripheral blood cell DNA methylation levels.176 However, the usage of biologicals provides therapeutic options when conventional approaches fail.
Allergen‐specific immunotherapy. The only causal treatment in allergic asthma is AIT, the role of which has been largely explored in recent clinical and experimental research. In a large cohort study involving 39 167 asthmatic subjects, a remarkable reduction of symptomatic medication was observed among 4111 patients treated by AIT during an 8‐year follow‐up. This outcome, which was mainly observed in young subjects, suggests that AIT might play a role in preventing progression from mild to more severe asthma.177 Moreover, AIT was shown to decrease serum IgE levels, while increasing serum levels of Der p 2‐specific IgG4 and Der p 2‐specific IgD in HDM‐sensitized asthmatic patients. The longitudinal change in Der p 2‐specific IgE/IgD ratio was similar to that in Der p 2‐specific IgE/IgG4 ratio. Asthma symptoms were improving during the AIT process, which also correlated with allergen-specific B‐cell responses.178
Many retrospective AIT studies investigated the risk of new‐onset asthma, the relative risk of medication dispensing for asthma and its potential progression among multimorbid allergic subjects. It was found that use of sublingual allergen immunotherapy tablets for pollen allergy for the treatment of AR could reduce the prevalence of asthma, decrease asthma medication usage, and also slow down the progression of asthma in comparison with a group receiving only symptomatic AR medication.179-181 Moreover, AIT with grass allergen peptide over 3 weeks before the beginning of grass pollen season was considered as an efficient and safe modality to protect patients with rhinoconjunctivitis and asthma from symptom onset.182 Additionally, it was suggested that in patients with both allergic asthma and AR, compared to standard care, sublingual allergen immunotherapy may have a cost-effective benefit with an incremental cost‐effectiveness ratio of £10,726 per quality‐adjusted life‐year which is nearly half of the (English) National Institute for Health and Clinical Excellence threshold.183
Studies have been continued to develop novel vaccines for allergen-specific immunotherapy. In an HDM‐driven allergic asthma mouse model, purified Der p1 and 2, similar to crude HDM extract, demonstrated a suppressive effect on AHR, eosinophilic inflammation, type 2 cytokines, and activation of lung structural cells.184 These optimal results based on purified natural allergens from animal studies suggest further research focusing on novel vaccines due to high demands in the field of allergic asthma.
A systematic review summarized recent updates about nonpharmacological asthma management. This review epitomized the methods used in studies, highlighted the importance of education and self‐management, and called for a thorough description of methods in future studies.185
10 CHALLENGES IN ASTHMA MANAGEMENT DURING COVID‐19 PANDEMICS
The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (COVID‐19) pandemics led to the worldwide emergency affecting the lives of more than 21 million people (status for 14.08.2020). It also raised a vigorous discussion in the research community, whether or not asthma should be classified as a COVID‐19 risk factor. Reports regarding asthma prevalence among COVID‐19 patients are inconsistent, varying from 0.9% in Wuhan to 17% in the United States.186 Wang et al exclude asthma as a factor influencing COVID‐19. Their statement is supported by the data from Avdeev et al and Zhang et al187, 188 Both groups did not observe the increase in the prevalence of bronchial asthma or COPD among patients with COVID‐19.187, 188 Likewise, in a cohort of 1504 severe asthmatics, Heffler et al. demonstrated that asthma patients are not at high risk of SARS‐CoV‐2 infection.189 Several more clinical studies ruled out asthma as COVID‐19‐predisposing factor.188,190,191 In the line with available reports, experts from 43 countries around the world in ARIA‐EAACI statement 186 do not consider asthma as a factor predisposing for SARS‐CoV‐2‐related disease. In contrast, another study advocates asthma as a COVID‐19 risk factor based on the impaired antiviral responses (interferon (IFN)‐α, IFN‐β, and IFN‐λ) that may predispose asthmatic patients to severe COVID‐19 manifestations.192 The US Center for Disease Control and Prevention also proposed that moderate‐to‐severe asthma should be considered a risk for severe COVID‐19 manifestations.186 Additionally, a recent study from Zhu et al (n = 492 768) demonstrated inflammatory phenotype of underlying asthma as a crucial factor for COVID‐19 risk assessment. They showed that nonallergic, but not allergic asthma predisposes patients to COVID‐19. Due to the reported discrepancies, available data should be interpreted with caution.193
Data are still unclear as the COVID‐19 population included in the studies is skewed toward older and hospitalized patients with predisposing comorbidities (such as hypertension, obesity, or diabetes) which are strong confounders.186,187 Additionally, early clinical reports regarding asthma prevalence among COVID‐19 cases often vary in terms of patients’ severity including those undergoing home quarantine,194 hos-pitalized,191,195 or referred to intensive care units and requiring mechanical ventilation.187 As a gender, smoking status, ethnicity, lifestyle, genetic background, and asthma phenotype can influence COVID‐19 outcomes, these factors should be carefully investigated.186, 195 Age seems especially significant, as childhood and adolescent asthma do not seem to be a hazard for COVID‐19.195 This may be related to reduced prevalence of comorbidities, lack of smoking, or boosted immune system due to recent vaccinations.195
Allergic responses accompanying asthma are hypothesized to play a protective role in the course of COVID‐19. Eosinopenia (decreased numbers of eosinophils in the blood) is a biomarker of severity and poor prognosis for COVID‐19 patients.195 Eosinophils respond to viral infections by releasing cytotoxic proteins, reactive oxygen species, and type 1 cytokine.195 Therefore, they play a supportive role in fighting the infection. Allergic patients present eosinophilia (increased numbers of eosinophils in the blood) and could have an advantage for the eosinophil‐ dependent antiviral responses.195 Notably, Licari et al. compared COVID‐19 children only with a large cohort (n = 120) of allergic children and concluded that allergic children had significantly higher frequencies of eosinophils in the blood.195
Receptor ACE2 and serine protease TMPRSS2 mediates SARS‐CoV‐2 entry into host cells.196-198 CD147, CD26, ANPEP, ENPEP, or DC‐SIGN are other receptors, proven or potentially involved in COVID‐19 pathogenesis.196 Radzikowska, Ding et al demonstrated that ACE2 and TMPRSS2 are expressed only in the epithelial tissues, whereas CD147 and its interaction partners are present in both epithelial tissues and immune cells.199 The expression of several SARS‐CoV‐2 receptors seems to be different in asthma patients (increased: TMPRSS2, CD44, ITGA6, NFATC2, NME1, and APOD; decreased: ACE2, ACE, MCT4, APH1A, S100A9, and NOD2). These findings were partially confirmed by others.186, 200 There is growing evidence that allergic-type 2 inflammation (mainly IL‐13) decreases ACE2 and increases TMPRSS2 expression.186,200 Additionally, ACE2 is an IFN‐induced gene.200 Intriguingly, the suppressed production of type I and III IFNs (IFN‐α/β and IFN‐λ) has been defined in severe atopic patients.191 During the viral defence, initial activation of innate immunity and IFN‐α/β and IFN‐λ are crucial to eliminating the virus. One can hypothesize that impaired/delayed type I and III IFN response observed in asthma patients, usually a foe, may play a protective role during SARS‐CoV‐2 infection and resulted in a subsequent decrease in an ACE2.200 Carli et al. suggested that the altered type I IFN response and the type 2 immunity signature in asthmatic patients downregulated the late‐phase hyper inflammation and consequently decreased tissue damage in the lungs, which might be beneficial in allergic asthma during COVID‐19.191 In contrast, the inhibition of type I IFNs is believed to play a crucial role in SARS‐CoV‐2 pathogenesis. Delayed IFN response and exaggerated inflammation might be related to increased virus replication, tissue damage, and severe COVID‐19 complications.201 Apparently, IFN response and its mechanisms during COVID‐19 represent a double‐edged sword and should be carefully investigated.
Asthma management in the times of COVID‐19 pandemics presented a challenge due to the overlap of the respiratory symptoms induced by asthma or by SARS‐CoV‐2 and by reduced face‐to‐face appointments and risky pulmonary function testing generating aerosols (Figure 4).186, 202-207 If possible, a video examination and attentive observation of the patient may help in proper diagnosis.202,208 Fever, persistent dry cough, flu‐like symptoms, and lack of wheeze can indicate COVID‐19 over asthma exacerbation.202 Severe asthma exacerbation leads to the hospitalization, which exposes asthmatics to unnecessary risk of being infected with SARS‐ CoV‐2.195 Steroid therapy in asthmatic patients in times of COVID‐19 pandemics should be continued as clinically indicated.187,192,200,202,205 Additionally, ICS use can be beneficial during COVID‐19 as it restores the impaired antiviral responses in asthma, subsequently leading to less severe manifestations.192 ICS suppresses coronavirus replication and decreases the ACE2/TMPRSS2 expression.200 However, ICS treatment can cause SARS‐CoV‐2 nebulization and its spread to the lower airways and surrounding surfaces.195 Therefore, the use of a spacer is encouraged.195 On the opposite, NICE guideline speaks against this perception stating that nebulizer‐ generated aerosols do not carry SARS‐CoV‐2 virus (www.nice.org.uk/ guidance/ng168). On the other hand, the use of OCS was linked with a risk of severe COVID‐19 manifestations (including death).186 If good asthma control in patients cannot be maintained, the introduction of azithromycin prophylaxis can be considered.192 Azithromycin reduces asthma exacerbations in patients, probably by augmenting IFN‐β and IFN‐λ responses, a subsequent decrease in viral replication, and reduced inflammatory response.192,200 Azithromycin interferes with the CD147 receptor and decreases its downstream signals after viral (rhinovirus) infections.200 COVID‐19 in high endemic HDM environment may affect antiviral immune responses. In a study by Akbarshahi et al., the authors hypothesized that HDM may adversely affect viral stimulus-induced antiviral IFN (types I and III) responses. To test the hypothesis, the authors investigated the effects of HDM exposure in both human bronchial epithelial cells and a mouse model of asthma exacerbation. They observed that Toll‐like receptor‐3 is the main target involved in reducing the IFN‐β and IFN‐λ responses by HDM.209
Lommatzsch et al reported the safety of omalizumab in a 52‐year‐old severe allergic asthmatic patient with SARS‐CoV‐2 infection.194 During the SARS‐CoV‐2 infection, the patient did not report any asthma exacerbation and maintained proper asthma control. A mild manifestation of COVID‐19 observed in this study might have been related to either (a) allergic endotype of underlying asthma, (b) ongoing omalizumab treatment, or (c) both.194 However, this observation needs further confirmation in the bigger patients’ population. Furthermore, omalizumab decreases the duration of rhinovirus infections and virus‐related exacerbations.192, 200 It can be hypothesized that anti‐IgE therapy protects also during SARS‐CoV‐2 infection. Experts suggest that immunomodulatory biological treatment strategies should continue in SARS‐CoV‐2‐negative asthmatic individuals in times of pandemics.186, 195, 200 However, due to possible immunosuppressive effects, they should be suspended in patients which developed COVID‐19, until disease resolution.186, 195
In summary, available data suggest asthma is not a risk factor for the development of severe forms of COVID‐19. Yet, COVID‐19 can be a severe disease in already damaged lungs of chronic asthma, particularly, ACOS and COPD patients. More evidence is needed to fully understand the impact of asthma and asthma therapies on the prevalence and the course of COVID‐19. More data are required from controlled clinical trials. Meanwhile, considering the current emergency, asthmatic patients should avoid SARS‐CoV‐2 infection and should receive the SARS‐CoV‐2 vaccine with priority, as recommended for influenza and the pneumococcal vaccine. In the present situation, prevention and proper asthma control, including the continuation of the background controller treatment, are the most efficacious way to assure the safety of asthma patients.210
11 CONCLUSION
Asthma research produces up to 9000 publications per year and represents one of the most rapidly developing areas. Most of the novel developments of the last year focus in the areas of precision medicine, endotypes and phenotypes, biomarkers, novel treatments such as biologicals, and real‐life studies. The stratified/personalized approach to patients and targeted treatments represent the future in many chronic diseases. A better understanding of the molecular mechanisms and discovering novel biomarkers represents the main target areas for further improving patient care.
CONFLICT OF INTEREST
Lacin Cevhertas, Ismail Ogulur, Debbie J. Maurer, Daniel Burla, Mei Ding, Kirstin Jansen, Jana Koch, Chengyao Liu, Siyuan Ma, Yasutaka Mitamura, Yaqi Peng, Urszula Radzikowska, Arturo O. Rinaldi, Pattraporn Satitsuksanoa, Anna Globinska Willem van de Veen, Milena Sokolowska, Katja Baerenfaller, Ya‐dong Gao, and Mübeccel Akdis declare no conflict of interest. Ioana Agache reports and Allergy journal Associate Editor Cezmi Akdis reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission’s Horison’s 2020 Framework Programme, Cure, Novartis Research Institutes, AstraZeneca, Scibase, GlaxoSmithKline, and other from Sanofi & Regeneron.
REFERENCES
1. Chung KF, Adcock IM. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy 2019; 74(9): 1649– 1659. Wiley Online Library CAS PubMed Web of Science® Google Scholar
2. Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019; 129(4): 1493– 1503. Crossref PubMed Web of Science®Google Scholar
3. Olafsdottir TA, Theodore F, Bjarnadottir K, et al. Eighty‐eight variants highlight the role of T cell regulation and airway remodelling in asthma pathogenesis. Nat Commun. 2020; 11(1): 393. CAS PubMed Web of Science®Google Scholar
4. Guo Y, Moon J‐Y, Laurie CC, et al. Genetic predisposition to obesity is associated with asthma in US Hispanics/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Allergy 2018; 73(7): 1547– 1550. Wiley Online Library CAS PubMed Web of Science® Google Scholar
5. D’Amato G, Annesi‐Maesano I, Cecchi L, D’Amato M. Latest news on the relationship between thunderstorms and respiratory allergy, severe asthma, and deaths for asthma. Allergy 2019; 74(1): 9– 11. Wiley Online Library PubMed Web of Science® Google Scholar
6. Kim J, Kim Y‐C, Ham J, et al. The effect of air pollutants on airway innate immune cells in patients with asthma. Allergy 2020; 75(9): 2372– 2376. Wiley Online Library PubMed Web of Science®Google Scholar
7. Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020; 145(6): 1517– 1528. Crossref CAS PubMed Web of Science® Google Scholar
8. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. The Lancet. 2018; 391(10122): 783– 800. Crossref PubMed Web of Science®Google Scholar 9. Borna E, Nwaru BI, Bjerg A, et al. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019; 74(9): 1703– 1715. Wiley Online Library PubMed Web of Science®Google Scholar
10. Branco PTBS, Alvim‐Ferraz MCM, Martins FG, Ferraz C, Vaz LG, Sousa SIV. Asthma in urban and rural pre-and primary school children according to the latest GINA definition. Allergy 2020; 75(7): 1771– 1776. Wiley Online Library PubMed Web of Science®Google Scholar
11. Liu X, Andersen SL, Olsen J, et al. Maternal hypothyroidism in the perinatal period and childhood asthma in the offspring. Allergy 2018; 73(4): 932– 939. Wiley Online Library CAS PubMed Web of Science®Google Scholar
12. Odling M, Andersson N, Ekstrom S, Melen E, Bergstrom A, Kull I. Characterization of asthma in the adolescent population. Allergy 2018; 73(8): 1744– 1746. Wiley Online Library CAS PubMed Web of Science®Google Scholar
13. Keller T, Hohmann C, Standl M, et al. The sex‐shift in single disease and multimorbid asthma and rhinitis during puberty – a study by MeDALL. Allergy 2018; 73(3): 602– 614. Wiley Online Library CAS PubMed Web of Science® Google Scholar
14. Farzan N, Vijverberg SJ, Hernandez‐ Pacheco N, et al. 17q21 variant increases the risk of exacerbations in asthmatic children despite inhaled corticosteroids use. Allergy 2018; 73(10): 2083– 2088. Wiley Online Library CAS PubMed Web of Science®Google Scholar
15. Gorlanova O, Illi S, Toncheva AA, et al. Protective effects of breastfeeding on respiratory symptoms in infants with 17q21 asthma risk variants. Allergy 2018; 73(12): 2388– 2392. Wiley Online Library CAS PubMed Web of Science®Google Scholar
16. Hur GY, Pham A, Miller M, et al. ORMDL3 but not neighbouring 17q21 gene LRRC3C is expressed in human lungs and lung cells of asthmatics. Allergy 2020; 75(8): 2061– 2065. Wiley Online Library PubMed Web of Science®Google Scholar
17. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019; 7(4): 358– 364. Crossref PubMed Web of Science® Google Scholar
18. Krautenbacher N, Flach N, Böck A, et al. A strategy for high‐dimensional multivariable analysis classifies childhood asthma phenotypes from genetic, immunological, and environmental factors. Allergy 2019; 74(7): 1364– 1373. Wiley Online Library CAS PubMed Web of Science ®Google Scholar
19. Dijk FN, Vijverberg SJ, Hernandez‐Pacheco N, et al. IL1RL1 gene variations are associated with asthma exacerbations in children and adolescents using inhaled corticosteroids. Allergy 2020; 75(4): 984– 989. Wiley Online Library PubMed Web of Science®Google Scholar
20. Agache I, Miller R, Gern JE, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy 2019; 74(3): 449– 463. Wiley Online Library PubMed Web of Science® Google Scholar
21. Yang S‐I, Lee S‐Y, Kim H‐B, et al. Prenatal particulate matter affects new asthma via airway hyperresponsiveness in school children. Allergy 2019; 74(4): 675– 684. Wiley Online Library PubMed Web of Science®Google Scholar
22. Paciência I, Cavaleiro Rufo J, Silva D, et al. Exposure to indoor endocrine‐disrupting chemicals and childhood asthma and obesity. Allergy 2019; 74(7): 1277– 1291. Wiley Online Library CAS PubMed Web of Science®Google Scholar
23. Brandt EB, Bolcas PE, Ruff BP, Khurana Hershey GK. IL33 contributes to diesel pollution‐mediated increase in experimental asthma severity. Allergy 2020; 75(9): 2254– 2266. Wiley Online Library CAS PubMed Web of Science®Google Scholar
24. Agache I, Annesi‐Maesano I, Bonertz A, et al. Prioritizing research challenges and funding for allergy and asthma and the need for translational research–The European strategic forum on allergic diseases. Allergy 2019; 74(11): 2064– 2076. Wiley Online Library PubMed Web of Science®Google Scholar
25. Weng C‐M, Wang C‐H, Lee M‐J, et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium‐derived cytokines expression in severe allergic asthma. Allergy 2018; 73(11): 2192– 2204. Wiley Online Library CAS PubMed Web of Science®Google Scholar
26. Wang E, Liu X, Tu W, et al. Benzo(a)pyrene facilitates dermatophagoides group 1 (Der f 1)‐induced epithelial cytokine release through aryl hydrocarbon receptor in asthma. Allergy 2019; 74(9): 1675– 1690. Wiley Online Library CAS PubMed Web of Science®Google Scholar
27. Gao Z, Fu WY, Sun Y, et al. Artemisia pollen allergy in China: component‐resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy 2019; 74(2): 284– 293. Wiley Online Library CAS PubMed Web of Science®Google Scholar
28. Hernández‐Ramírez G, Pazos‐Castro D, Gómez Torrijos E, et al. Group 1 allergens, transported by mold spores, induce asthma exacerbation in a mouse model. Allergy 2020; 75(9): 2388– 2391. Wiley Online Library CAS PubMed Web of Science®Google Scholar
29. Watai K, Fukutomi Y, Hayashi H, et al. De novo sensitization to Aspergillus fumigatus in adult asthma over a 10‐year observation period. Allergy 2018; 73(12): 2385– 2388. Wiley Online Library CAS PubMed Web of Science®Google Scholar
30. Sullivan A, Hunt EB, Ward C, et al. The presence of Aspergillus fumigatus in asthmatic airways is not clearly related to clinical disease severity. Allergy 2020; 75(5): 1146– 1154. Wiley Online Library CAS PubMed Web of Science® Google Scholar
31. Hew M, Lee J, Susanto NH, et al. The 2016 Melbourne thunderstorm asthma epidemic: Risk factors for severe attacks requiring hospital admission. Allergy 2019; 74(1): 122– 130. Wiley Online Library PubMed Web of Science®Google Scholar
32. Hew M, Lee J, Varese N, et al. Epidemic thunderstorm asthma susceptibility from sensitization to ryegrass (Lolium perenne) pollen and major allergen Lol p 5. Allergy 2020; 75(9): 2369– 2372. Wiley Online Library CAS PubMed Web of Science®Google Scholar
33. Erbas B, Jazayeri M, Lambert KA, et al. Outdoor pollen is a trigger of child and adolescent asthma emergency department presentations: A systematic review and meta‐analysis. Allergy 2018; 73(8): 1632– 1641. Wiley Online Library CAS PubMed Web of Science®Google Scholar
34. Kim SY, Sim S, Choi HG. Active and passive smoking impacts on asthma with quantitative and temporal relations: A Korean Community Health Survey. Sci Rep. 2018; 8(1): 8614. PubMed Web of Science®Google Scholar
35. McAlinden KD, Naidu V, Sohal SS, Sharma P. In utero exposure to nicotine-containing electronic cigarettes increase the risk of allergic asthma in female offspring. Am J Physiol Lung Cell Mol Physiol 2020; 319(2): L197– L402. PubMed Web of Science®Google Scholar
36. Eddy RL, Serajeddini H, Knipping D, et al. Pulmonary functional MRI and CT in a survivor of bronchiolitis and respiratory failure caused by e‐cigarette use. Chest 2020; 158(4): e147– e151. Crossref CAS PubMed Web of Science®Google Scholar
37. Adkins SH, Anderson KN, Goodman AB, et al. Demographics, substance use behaviours, and clinical characteristics of adolescents with e‐Cigarette, or vaping, product use‐associated lung injury (EVALI) in the United States in 2019. JAMA Pediatr. 2020; 174(7):e200756. Crossref PubMed Web of Science®Google Scholar
38. Werner AK, Koumans EH, Chatham‐ Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020; 382(17): 1589– 1598. CAS PubMed Web of Science®Google Scholar
39. Chung SJ, Kim B‐K, Oh JH, et al. Novel tobacco products including electronic cigarette and heated tobacco products increase risk of allergic rhinitis and asthma in adolescents: Analysis of Korean youth survey. Allergy 2020; 75(7): 1640– 1648. Wiley Online Library CAS PubMed Web of Science®Google Scholar
40. Venter C, Meyer RW, Nwaru BI, et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019; 74(8): 1429– 1444. Wiley Online Library PubMed Web of Science®Google Scholar
41. Venter C, Greenhawt M, Meyer RW, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy 2020; 75(3): 497– 523. Wiley Online Library PubMed Web of Science®Google Scholar
42. Kang S‐Y, Song W‐J, Kim M‐H, et al. Dietary assessment and the development of asthma in Korean adolescents and adults. Allergy 2018; 73(11): 2254– 2256. Wiley Online Library PubMed Web of Science®Google Scholar
43. Comberiati P, Peroni DG. Vitamin D supplementation in pregnancy does not prevent school‐age asthma. Allergy 2020; 75(8): 2143– 2144. Wiley Online Library PubMed Web of Science®Google Scholar
44. Litonjua AA, Carey VJ, Laranjo N, et al. Six‐year follow‐up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020; 382(6): 525– 533. Crossref PubMed Web of Science® Google Scholar
45. Hennessy Á, Hourihane JO’B, Malvisi L, et al. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE Birth Cohort Study. Allergy 2018; 73(11): 2182– 2191. Wiley Online Library CAS PubMed Web of Science® Google Scholar
46. Tomita Y, Fukutomi Y, Irie M, et al. acid-suppressive medication as a possible risk factor for late‐onset asthma. Allergy 2020; 75(5): 1247– 1250. Wiley Online Library PubMed Web of Science®Google Scholar
47. Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity 2020; 52(2): 241– 255. Crossref CAS PubMed Web of Science®Google Scholar
48. Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: How early‐life environmental exposures shape gut microbiome and influence the development of asthma and allergic disease. Allergy 2019; 74(11): 2103– 2115. Wiley Online Library PubMed Web of Science®Google Scholar
49. Walter J, O’Mahony L. The importance of social networks‐An ecological and evolutionary framework to explain the role of microbes in the aetiology of allergy and asthma. Allergy 2019; 74(11): 2248– 2251. Wiley Online Library PubMed Web of Science®Google Scholar
50. Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018; 9(1): 141. Crossref PubMed Web of Science®Google Scholar
51. Bannier MAGE, Best N, Bervoets L, et al. Gut microbiota in wheezing preschool children and the association with childhood asthma. Allergy 2020; 75(6): 1473– 1476. Wiley Online Library PubMed Web of Science®Google Scholar
52. Lee J‐J, Kim S‐H, Lee M‐J, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy 2019; 74(4): 709– 719. Wiley Online Library CAS PubMed Web of Science®Google Scholar
53. Kim B‐S, Lee E, Lee M‐J, et al. Different functional genes of upper airway microbiome associated with the natural course of childhood asthma. Allergy 2018; 73(3): 644– 652. Wiley Online Library CAS PubMed Web of Science® Google Scholar
54. Spacova I, Petrova MI, Fremau A, et al. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen‐induced allergic asthma in a murine model. Allergy 2019; 74(1): 100–110. Wiley Online Library CAS PubMed Web of Science®Google Scholar
55. Roßberg S, Keller T, Icke K, et al. Orally applied bacterial lysate in infants at risk for atopy does not prevent atopic dermatitis, allergic rhinitis, asthma or allergic sensitization at school age: Follow‐up of a randomized trial. Allergy 2020; 75(8): 2020– 2025. Wiley Online Library PubMed Web of Science®Google Scholar
56. Michalovich D, Rodriguez‐Perez N, Smolinska S, et al. Obesity and disease severity magnify disturbed microbiome‐ immune interactions in asthma patients. Nat Commun. 2019; 10(1): 5711. Crossref CAS PubMed Web of Science® Google Scholar
57. Alhasan MM, Cait AM, Heimesaat MM, et al. Antibiotic use during pregnancy increases offspring asthma severity in a dose‐dependent manner. Allergy 2020; 75(8): 1979– 1990. Wiley Online Library PubMed Web of Science®Google Scholar
58. Eguiluz‐Gracia I, Mathioudakis AG, Bartel S, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020; 75(9): 2170– 2184. Wiley Online Library PubMed Web of Science®Google Scholar
59. Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI, Luster AD. Distinct functions of tissue‐resident and circulating memory Th2 cells in allergic airway disease. J Exp Med. 2020; 217(9).10.1084/jem.20190 865. PubMed Web of Science®Google Scholar
60. Vieira Braga FA, Kar G, Berg M, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019; 25(7): 1153–1163. Crossref CAS PubMed Web of Science® Google Scholar
61. Asayama K, Kobayashi T, D’Alessandro‐ Gabazza CN, et al. Protein S protects against allergic bronchial asthma by modulating Th1/Th2 balance. Allergy 2020; 75(9): 2267– 2278. Wiley Online Library CAS PubMed Web of Science® Google Scholar
62. Bolcas PE, Brandt EB, Ruff BP, Kalra M, Khurana Hershey GK. Cysteamine prevents asthma development and reduces airway hyperresponsiveness in experimental asthma. Allergy 2020; 75: 2675–2677. https://doi.org/10.1111/all. 14332 Wiley Online Library CAS PubMed Web of Science®Google Scholar
63. Lu Y, Kared H, Tan SW, et al. Dynamics of helper CD4 T cells during acute and stable allergic asthma. Mucosal Immunol. 2018; 11(6): 1640– 1652. Crossref CAS PubMed Web of Science® Google Scholar
64. Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non‐T2 asthma. Allergy 2020; 75(2): 311– 325. Wiley Online Library PubMed Web of Science®Google Scholar
65. Worth L, Michel S, Gaertner VD, Kabesch M, Schieck M. Asthma‐ and IgE‐associated polymorphisms affect the expression of TH 17 genes. Allergy 2018; 73(6): 1342– 1347. Wiley Online Library CAS PubMed Web of Science®Google Scholar
66. Ro M, Kwon SY, Kim JH. Leukotriene B4 receptors mediate the production of IL‐17, thus contributing to neutrophil‐ dominant asthmatic airway inflammation. Allergy 2019; 74(9): 1797– 1799. Wiley Online Library PubMed Web of Science® Google Scholar
67. Ekstedt S, Stenberg H, Tufvesson E, et al. The potential role of CD16(high) CD62L(dim) neutrophils in allergic asthma. Allergy 2019; 74(11): 2265– 2268. Wiley Online Library PubMed Web of Science®Google Scholar
68. Brown GC, Vilalta A, Fricker M. Phagoptosis – cell death by phagocytosis ‐ plays central roles in physiology, host defence and pathology. Curr Mol Med 2015; 15(9): 842–851. CAS PubMed Web of Science® Google Scholar
69. Ekstedt S, Tufvesson E, Bjermer L, Kumlien Georén S, Cardell LO. A new role for “eat me” and “don’t eat me” markers on neutrophils in asthmatic airway inflammation. Allergy 2020; 75(6): 1510– 1512. Wiley Online Library PubMed Web of Science® Google Scholar
70. Ferreira DS, Carvalho‐Pinto RM, Gregório MG, et al. Airway pathology in severe asthma is related to airflow obstruction but not symptom control. Allergy 2018; 73(3): 635– 643. Wiley Online Library CAS PubMed Web of Science®Google Scholar
71. Altman MC, Gill MA, Whalen E, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol. 2019; 20(5): 637– 651. Crossref CAS PubMed Web of Science®Google Scholar
72. Hasegawa K, Hoptay CE, Harmon B, et al. Association of type 2 cytokines in severe rhinovirus bronchiolitis during infancy with the risk of developing asthma: a multicenter prospective study. Allergy 2019; 74(7): 1374– 1377. Wiley Online Library PubMed Web of Science®Google Scholar
73. Lund RJ, Osmala M, Malonzo M, et al. Atopic asthma after rhinovirus‐induced wheezing is associated with DNA methylation change in the SMAD3 gene promoter. Allergy 2018; 73(8): 1735– 1740. Wiley Online Library CAS PubMed Web of Science®Google Scholar
74. Schuler CF, Malinczak C‐A, Best SKK, et al. Inhibition of uric acid or IL‐1β ameliorate respiratory syncytial virus immunopathology and development of asthma. Allergy 2020; 75(9): 2279– 2293. Wiley Online Library CAS PubMed Web of Science® Google Scholar
75. Törmänen S, Lauhkonen E, Riikonen R, et al. Risk factors for asthma after infant bronchiolitis. Allergy 2018; 73(4): 916– 922. Wiley Online Library CAS PubMed Web of Science®Google Scholar
76. Schwarze J, Openshaw P, Jha A, et al. Influenza burden, prevention, and treatment in asthma‐A scoping review by the EAACI Influenza in the asthma task force. Allergy 2018; 73(6): 1151– 1181. Wiley Online Library CAS PubMed Web of Science®Google Scholar
77. Suojalehto H, Lindstrom I, Wolff H, Puustinen A. Nasal protein profiles in work‐related asthma caused by different exposures. Allergy 2018; 73(3): 653– 663. Wiley Online Library CAS PubMed Web of Science®Google Scholar
78. Felix MMR, Kuschnir FC. Arginase inhibitors: An alternative in the treatment of obese asthma? Allergy 2020; 75(6): 1525– 1526. Wiley Online Library PubMed Web of Science®Google Scholar
79. Meurs H, Zaagsma J, Maarsingh H, van Duin M. Recent patents in allergy/ immunology: use of arginase inhibitors in the treatment of asthma and allergic rhinitis. Allergy 2019; 74(6): 1206– 1208. Wiley Online Library PubMed Web of Science®Google Scholar
80. Meurs H, Zaagsma J, Maarsingh H, van Duin M. Reply to: “Arginase inhibitors: An alternative in the treatment of obese asthma?”. Allergy 2020; 75(6): 1527– 1528. Wiley Online Library PubMed Web of Science®Google Scholar
81. Khumalo J, Kirstein F, Scibiorek M, Hadebe S, Brombacher F. Therapeutic and prophylactic deletion of IL‐4Ra‐signaling ameliorates established ovalbumin-induced allergic asthma. Allergy 2020; 75(6): 1347– 1360. Wiley Online Library CAS PubMed Web of Science®Google Scholar
82. Ro M, Lee AJ, Kim JH. 5‐/12‐Lipoxygenase ‐linked cascade contributes to the IL‐33‐induced synthesis of IL‐13 in mast cells, thus promoting asthma development. Allergy 2018; 73(2): 350– 360. Wiley Online Library CAS PubMed Web of Science®Google Scholar
83. Lv J, Xiong Y, Li W, et al. IL‐37 inhibits IL‐4/IL‐13‐induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy 2018; 73(8): 1642– 1652. Wiley Online Library CAS PubMed Web of Science®Google Scholar
84. Russkamp D, Aguilar‐Pimentel A, Alessandrini F, et al. IL‐4 receptor α blockade prevents sensitization and alters acute and long‐lasting effects of allergen‐specific immunotherapy of murine allergic asthma. Allergy 2019; 74(8): 1549– 1560. Wiley Online Library CAS PubMed Web of Science®Google Scholar
85. Hong JY, Kim M, Sol IS, et al. Chitotriosidase inhibits allergic asthmatic airways via regulation of TGF‐β expression and Foxp3. Allergy 2018; 73(8): 1686– 1699. Wiley Online Library CAS PubMed Web of Science®Google Scholar
86. Guan Q, Yang B, Warrington RJ, et al. Myeloid‐derived suppressor cells: roles and relations with Th2, Th17, and Treg cells in asthma. Allergy 2019; 74(11): 2233–2237. Wiley Online Library PubMed Web of Science®Google Scholar
87. Wirz OF, Głobińska A, Ochsner U, et al. Comparison of regulatory B cells in asthma and allergic rhinitis. Allergy 2019; 74(4): 815– 818. Wiley Online Library PubMed Web of Science®Google Scholar
88. Brightling CE, Brusselle G, Altman P. The impact of the prostaglandin D2 receptor 2 and its downstream effects on the pathophysiology of asthma. Allergy 2020; 75(4): 761– 768. Wiley Online Library CAS PubMed Web of Science®Google Scholar
89. Moon H‐G, Kim S‐J, Lee MK, et al. Colony‐stimulating factor 1 and its receptor are new potential therapeutic targets for allergic asthma. Allergy 2020; 75(2): 357– 369. Wiley Online Library CAS PubMed Web of Science®Google Scholar
90. Kim S‐H, Jung H‐W, Kim M, et al. Ceramide/sphingosine‐1‐phosphate imbalance is associated with distinct inflammatory phenotypes of uncontrolled asthma. Allergy 2020; 75(8): 1991– 2004. Wiley Online Library PubMed Web of Science®Google Scholar
91. Choi Y, Kim M, Kim SJ, Yoo H‐J, Kim S‐H, Park H‐S. Metabolic shift favouring C18:0 ceramide accumulation in obese asthma. Allergy 2020;75:2858–2866. https:// doi.org/10.1111/all.14366 Wiley Online Library CAS PubMed Web of Science® Google Scholar
92. Asaduzzaman M, Davidson C, Nahirney D, Fiteih Y, Puttagunta L, Vliagoftis H. Proteinase‐activated receptor‐2 blockade inhibits changes seen in a chronic murine asthma model. Allergy 2018; 73(2): 416– 420. Wiley Online Library CAS PubMed Web of Science® Google Scholar
93. Yu QN, Guo YB, Li X, et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via the STAT pathway in patients with asthma. Allergy 2018; 73(9): 1860– 1870. Wiley Online Library CAS PubMed Web of Science®Google Scholar
94. Milara J, Morell A, de Diego A, Artigues E, Morcillo E, Cortijo J. Mucin 1 deficiency mediates corticosteroid insensitivity in asthma. Allergy 2019; 74(1): 111– 121. Wiley Online Library CAS PubMed Web of Science®Google Scholar
95. Bartel S, La Grutta S, Cilluffo G, et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2020; 75(2): 346– 356. Wiley Online Library CAS PubMed Web of Science®Google Scholar
96. Uwadiae FI, Pyle CJ, Walker SA, Lloyd CM, Harker JA. Targeting the ICOS/ICOS‐L pathway in a mouse model of established allergic asthma disrupts T follicular helper cell responses and ameliorates disease. Allergy 2019; 74(4): 650– 662. Wiley Online Library CAS PubMed Web of Science®Google Scholar
97. Bateman ED, Reddel HK, van Zyl‐Smit RN, Agusti A. The asthma‐COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015; 3(9): 719– 728. Crossref PubMed Web of Science®Google Scholar
98. Wang YC, Jaakkola MS, Lajunen TK, Lai CH, Jaakkola JJK. Asthma‐COPD Overlap Syndrome among subjects with newly diagnosed adult‐onset asthma. Allergy 2018; 73(7): 1554– 1557. Wiley Online Library PubMed Web of Science®Google Scholar
99. Campo P, Eguiluz‐Gracia I, Plaza‐Serón MC, et al. Bronchial asthma triggered by house dust mites in patients with local allergic rhinitis. Allergy 2019; 74(8): 1502– 1510. Wiley Online Library CAS PubMed Web of Science®Google Scholar
100. Toppila‐Salmi S, Chanoine S, Karjalainen J, Pekkanen J, Bousquet J, Siroux V. Risk of adult‐onset asthma increases with the number of allergic multimorbidities and decreases with age. Allergy 2019; 74(12): 2406– 2416. Wiley Online Library PubMed Web of Science®Google Scholar
101. Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy 2018; 73(5): 993– 1002. Wiley Online Library CAS PubMed Web of Science® Google Scholar
102. Sharma N, Akkoyunlu M, Rabin RL. Macrophages‐common culprit in obesity and asthma. Allergy 2018; 73(6): 1196– 1205. Wiley Online Library CAS PubMed Web of Science®Google Scholar
103. Chen YC, Fan HY, Yang C, Lee YL. Early pubertal maturation and risk of childhood asthma: a Mendelian randomization and longitudinal study. Allergy 2020; 75(4): 892– 900. Wiley Online Library CAS PubMed Web of Science®Google Scholar
104. Skaaby T, Taylor AE, Thuesen BH, et al. Estimating the causal effect of body mass index on hay fever, asthma and lung function using Mendelian randomization. Allergy 2018; 73(1): 153– 164. Wiley Online Library CAS PubMed Web of Science®Google Scholar
105. Tay TR, Hew M. Comorbid, “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy 2018; 73(7): 1369– 1382. Wiley Online Library CAS PubMed Web of Science® Google Scholar
106. Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019; 74(12): 2312– 2319. Wiley Online Library PubMed Web of Science®Google Scholar
107. Li Y, Jiang Q, Ji Y, Cao C. Anxiety and depression may associate with poorer control and quality of life in adults with asthma. Allergy 2020; 75(7): 1759– 1762. Wiley Online Library PubMed Web of Science®Google Scholar
108.Lemonnier N, Melén E, Jiang Y, et al. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy 2020; 75: 3248– 3260. https://doi.org/10.1111/all.14314 Wiley Online Library Web of Science® Google Scholar
109. Siroux V, Ballardini N, Soler M, et al. The asthma‐rhinitis multimorbidity is associated with IgE polysensitization in adolescents and adults. Allergy 2018; 73(7): 1447– 1458. Wiley Online Library CAS PubMed Web of Science®Google Scholar
110. Lemmetyinen RE, Karjalainen JV, But A, et al. Higher mortality of adults with asthma: a 15‐year follow‐up of a population‐based cohort. Allergy 2018; 73(7): 1479– 1488. Wiley Online Library CAS PubMed Web of Science®Google Scholar
111. Postma DS, Brightling C, Baldi S, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019; 7(5): 402– 416. Crossref PubMed Web of Science®Google Scholar
112. Shirai T, Akamatsu T, Hirai K, et al. Oscillometry improves earlier than spirometry after benralizumab initiation in severe asthma. Allergy 2020; 75: 2678– 2680. https://doi.org/ 10.1111/all.14339 Wiley Online Library CAS PubMed Web of Science®Google Scholar
113. Dunn RM, Busse PJ, Wechsler ME. Asthma in the elderly and late‐onset adult asthma. Allergy 2018; 73(2): 284– 294. Wiley Online Library CAS PubMed Web of Science®Google Scholar
114. Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID‐ exacerbated respiratory disease (N‐ERD)‐a EAACI position paper. Allergy 2019; 74(1): 28– 39. Wiley Online Library PubMed Web of Science® Google Scholar
115. Diamant Z, Vijverberg S, Alving K, et al. Toward clinically applicable biomarkers for asthma: An EAACI position paper. Allergy 2019; 74(10): 1835– 1851. Wiley Online Library PubMed Web of Science®Google Scholar
116.Celejewska‐Wójcik N, Wójcik K, Ignacak‐Popiel M, et al. Subphenotypes of nonsteroidal antiinflammatory disease‐ exacerbated respiratory disease identified by latent class analysis. Allergy 2020; 75(4): 831– 840. Wiley Online Library CAS PubMed Web of Science®Google Scholar
117. Mastalerz L, Tyrak KE, Ignacak M, et al. Prostaglandin E2 decrease in induced sputum of hypersensitive asthmatics during oral challenge with aspirin. Allergy 2019; 74(5): 922– 932. Wiley Online Library CAS PubMed Web of Science® Google Scholar
118. Chen LC, Tseng HM, Kuo ML, et al. A composite of exhaled LTB4, LXA4, FeNO, and FEV1 as an “asthma classification ratio” characterizes childhood asthma. Allergy 2018; 73(3): 627– 634. Wiley Online Library CAS PubMed Web of Science®Google Scholar
119. Tanaka H, Nakatani E, Fukutomi Y, et al. Identification of patterns of factors preceding severe or life‐threatening asthma exacerbations in a nationwide study. Allergy 2018; 73(5): 1110– 1118. Wiley Online Library CAS PubMed Web of Science®Google Scholar
120. Jutel M, Brüggenjürgen B, Richter H, Vogelberg C. Real‐world evidence of subcutaneous allergoid immunotherapy in house dust mite‐induced allergic rhinitis and asthma. Allergy 2020; 75(8): 2050– 2058. Wiley Online Library PubMed Web of Science®Google Scholar
121.Suarthana E, Taghiakbari M, Saha‐Chaudhuri P, et al. The validity of the Canadian clinical scores for occupational asthma in European populations. Allergy 2020; 75(8): 2124– 2126. Wiley Online Library PubMed Web of Science®Google Scholar
122. Vandenplas O, Godet J, Hurdubaea L, et al. Are high‐ and low‐molecular‐weight sensitizing agents associated with different clinical phenotypes of occupational asthma? Allergy 2019; 74(2): 261– 272. Wiley Online Library PubMed Web of Science®Google Scholar
123. Beretta C, Rifflart C, Evrard G, Jamart J, Thimpont J, Vandenplas O. Assessment of eosinophilic airway inflammation as a contribution to the diagnosis of occupational asthma. Allergy 2018; 73(1): 206– 213. Wiley Online Library CAS PubMed Web of Science®Google Scholar
124. Eguiluz‐Gracia I, Tay TR, Hew M, et al. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy 2018; 73(12): 2290– 2305. Wiley Online Library PubMed Web of Science®Google Scholar
125. Tan H‐T, Hagner S, Ruchti F, et al. Tight junction, mucin, and inflammasome‐ related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2019; 74(2): 294– 307. Wiley Online Library CAS PubMed Web of Science®Google Scholar
126. Boudier A, Chanoine S, Accordini S, et al. Data‐driven adult asthma phenotypes based on clinical characteristics are associated with asthma outcomes twenty years later. Allergy 2019; 74(5): 953– 963. Wiley Online Library PubMed Web of Science®Google Scholar
127. Ivanova O, Richards LB, Vijverberg SJ, et al. What did we learn from multiple omics studies in asthma? Allergy 2019; 74(11): 2129– 2145. Wiley Online Library PubMed Web of Science®Google Scholar
128. Su M‐W, Lin W‐C, Tsai C‐H, et al. Childhood asthma clusters reveal neutrophil‐ predominant phenotype with distinct gene expression. Allergy 2018; 73(10): 2024– 2032. Wiley Online Library CAS PubMed Web of Science®Google Scholar
129. Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75(5): 1023– 1042. Wiley Online Library CAS PubMed Web of Science®Google Scholar
130. Agache I, Rocha C, Beltran J, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: a systematic review for the EAACI Guidelines ‐ recommendations on the use of biologicals in severe asthma. Allergy 2020; 75(5): 1043– 1057. Wiley Online Library CAS PubMed Web of Science®Google Scholar
131. Agache I, Song Y, Rocha C, et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines‐Recommendations on the use of biologicals in severe asthma. Allergy 2020; 75(5): 1058– 1068. Wiley Online Library PubMed Web of Science®Google Scholar
132. Howarth P, Quirce S, Papi A, et al. Eosinophil‐derived neurotoxin and clinical outcomes with mepolizumab in severe eosinophilic asthma. Allergy 2020; 75(8): 2085– 2088. Wiley Online Library PubMed Web of Science®Google Scholar
133. Kuo CS, Pavlidis S, Zhu J, et al. Contribution of airway eosinophils in airway wall remodeling in asthma: Role of MMP‐10 and MET. Allergy 2019; 74(6): 1102– 1112. Wiley Online Library CAS PubMed Web of Science®Google Scholar
134. Sabogal Piñeros YS, Bal SM, Dijkhuis A, et al. Eosinophils capture viruses, a capacity that is defective in asthma. Allergy 2019; 74(10): 1898– 1909. Wiley Online Library CAS PubMed Web of Science®Google Scholar
135. Tarancon R, Uranga S, Martin C, Aguilo N. Mycobacterium tuberculosis infection prevents asthma and abrogates eosinophilopoiesis in an experimental model. Allergy 2019; 74(12): 2512– 2514. Wiley Online Library PubMed Web of Science®Google Scholar
136. Bagnasco D, Massolo A, Bonavia M, et al. The importance of being not significant: Blood eosinophils and clinical responses do not correlate in severe asthma patients treated with mepolizumab in real life. Allergy 2020; 75(6): 1460– 1463. Wiley Online Library PubMed Web of Science®Google Scholar
137. Jesenak M, Diamant Z. Blood eosinophils: In quest of a Holy Grail for personalized asthma treatment with biologicals. Allergy 2020; 75(6): 1294– 1297. Wiley Online Library PubMed Web of Science®Google Scholar
138. Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018; 73(2): 490– 497. Wiley Online Library CAS PubMed Web of Science® Google Scholar
139. Rodrigo‐Muñoz JM, Cañas JA, Sastre B, et al. Asthma diagnosis using integrated analysis of eosinophil microRNAs. Allergy 2019; 74(3): 507– 517.Wiley Online Library CAS PubMed Web of Science® Google Scholar
140. Granger V, Taillé C, Roach D, et al. Circulating neutrophil and eosinophil extracellular traps are markers of severe asthma. Allergy 2020; 75(3): 699– 702. Wiley Online Library PubMed Web of Science®Google Scholar
141. Beuraud C, Lombardi V, Luce S, et al. CCR10(+) ILC2s with ILC1‐like properties exhibit a protective function in severe allergic asthma. Allergy 2019; 74(5): 933– 943. Wiley Online Library CAS PubMed Web of Science®Google Scholar
142. Hoang JA, Mashouri P, Dai R, et al. Extract and component‐specific sensitization patterns in Canadian moderate‐to‐severe preschool asthmatics. Allergy 2019; 74(12): 2519– 2521. Wiley Online Library PubMed Web of Science®Google Scholar
143. Nieto‐Fontarigo JJ, González‐Barcala FJ, Andrade‐Bulos LJ, et al. iTRAQ‐based proteomic analysis reveals potential serum biomarkers of allergic and nonallergic asthma. Allergy 2020; 75: 3171– 3183. Wiley Online Library Web of Science®Google Scholar
144. Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open‐label, parallel‐group, randomised controlled trial. Lancet Resp Med. 2020; 8(7): 671– 680. Crossref CAS PubMed Web of Science®Google Scholar
145. Alvaro‐Lozano M, Akdis CA, Akdis M, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol. 2020; 31 Suppl 25(Suppl 25): 1– 101. Wiley Online Library PubMed Web of Science®Google Scholar
146. Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite‐driven allergic asthma. Allergy 2019; 74(5): 855– 873. Wiley Online Library CAS PubMed Web of Science®Google Scholar
147. Farraia M, Cavaleiro Rufo J, Paciência I, et al. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy 2020; 75(7): 1630– 1639. Wiley Online Library CAS PubMed Web of Science®Google Scholar
148. Abdel‐Aziz MI, de Vries R, Lammers A, et al. Cross‐sectional biomarker comparisons in asthma monitoring using a longitudinal design: The eNose premise. Allergy 2020; 75: 2690–2693. https://doi.org/ 10.1111/all.14354. Online ahead of print Wiley Online Library PubMed Web of Science®Google Scholar
149. Cavaleiro Rufo J, Paciência I, Mendes FC, et al. Exhaled breath condensate volatilome allows sensitive diagnosis of persistent asthma. Allergy 2019; 74(3): 527–534. Wiley Online Library CAS PubMed Web of Science®Google Scholar
150. Bousquet J, Hellings PW, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019; 143(3): 864– 879. Crossref PubMed Web of Science® Google Scholar
151. Bédard A, Antó JM, Fonseca JA, et al. Correlation between work impairment, scores of rhinitis severity and asthma using the MASK‐air(®) App. Allergy 2020; 75(7): 1672– 1688. Wiley Online Library CAS PubMed Web of Science®Google Scholar
152. Bousquet J, Arnavielhe S, Bedbrook A, et al. The allergic rhinitis and its impact on asthma (ARIA) score of allergic rhinitis using mobile technology correlates with quality of life: The MASK study. Allergy 2018; 73(2): 505– 510. Wiley Online Library CAS PubMed Web of Science® Google Scholar
153. Bousquet J, Agache I, Aliberti MR, et al. Transfer of innovation on allergic rhinitis and asthma multimorbidity in the elderly (MACVIA‐ARIA) ‐ EIP on AHA Twinning Reference Site (GARD research demonstration project). Allergy 2018; 73(1): 77– 92. Wiley Online Library CAS PubMed Web of Science®Google Scholar
154. Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet 2020; 395(10221): 371– 383. Crossref CAS PubMed Web of Science®Google Scholar
155. Fahy JV. Type 2 inflammation in asthma– present in most, absent in many. Nat Rev Immunol. 2015; 15(1): 57– 65. Crossref CAS PubMed Web of Science®Google Scholar
156. Akdis CA, Arkwright PD, Brüggen M‐C, et al. Type 2 immunity in the skin and lungs. Allergy 2020; 75(7): 1582– 1605. Wiley Online Library CAS PubMed Web of Science®Google Scholar
157. McDowell PJ, Heaney LG. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy 2020; 75(2): 302– 310. Wiley Online Library PubMed Web of Science®Google Scholar
158. Ho T, Al‐Selahi E, Mukherjee M, et al. Sputum and serum immunoglobulins in adult asthmatics with recurrent respiratory tract infections. Allergy 2020; 75(8): 2105– 2108. Wiley Online Library PubMed Web of Science®Google Scholar
159. Fieten KB, Rijssenbeek‐Nouwens LH, Hashimoto S, Bel EH, Weersink EJ. Less exacerbations and sustained asthma control 12 months after high altitude climate treatment for severe asthma. Allergy 2019; 74(3): 628– 630. Wiley Online Library PubMed Web of Science® Google Scholar
160. Fieten KB, Zijlstra WT, van Os‐Medendorp H, et al. Comparing high altitude treatment with current best care in Dutch children with moderate to severe atopic dermatitis (and asthma): study protocol for a pragmatic randomized controlled trial (DAVOS trial). Trials. 2014; 15: 94. Crossref PubMed Web of Science®Google Scholar
161. Boonpiyathad T, Capova G, Duchna H‐W, et al. Impact of high‐altitude therapy on type‐2 immune responses in asthma patients. Allergy 2020; 75(1): 84– 94. Wiley Online Library CAS PubMed Web of Science®Google Scholar
162. Seys SF, Quirce S, Agache I, et al. Severe asthma: entering an era of new concepts and emerging therapies: Highlights of the 4th international severe asthma forum, Madrid, 2018. Allergy 2019; 74(11): 2244– 2248. Wiley Online Library PubMed Web of Science®Google Scholar
163. Eyerich S, Metz M, Bossios A, Eyerich K. New biological treatments for asthma and skin allergies. Allergy 2020; 75(3): 546– 560. Wiley Online Library PubMed Web of Science®Google Scholar
164. Voorham J, Xu X, Price DB, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy 2019; 74(2): 273– 283. Wiley Online Library PubMed Web of Science®Google Scholar
165. Papadopoulos NG, Barnes P, Canonica GW, et al. The evolving algorithm of biological selection in severe asthma. Allergy 2020; 75(7): 1555– 1563. Wiley Online Library PubMed Web of Science® Google Scholar
166. Israel E, Reddel HK. Severe and Difficult ‐to‐Treat Asthma in Adults. N Engl J Med. 2017; 377(10): 965– 976. Crossref CAS PubMed Web of Science®Google Scholar
167.Chapman KR, Albers FC, Chipps B, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy 2019; 74(9): 1716– 1726. Wiley Online Library CAS PubMed Web of Science®Google Scholar
168. Mukherjee M, Bakakos P, Loukides S. New paradigm in asthma management: Switching between biologics!. Allergy 2020; 75(4): 743– 745. Wiley Online Library PubMed Web of Science®Google Scholar
169. Deschildre A, Roussel J, Drumez E, et al. Omalizumab discontinuation in children with severe allergic asthma: an observational real‐life study. Allergy 2019; 74(5): 999– 1003. Wiley Online Library PubMed Web of Science®Google Scholar
170. Mathur SK, Modena BD, Coumou H, Barker P, Kreindler JL, Zangrilli JG. Postbronchodilator lung function improvements with benralizumab for patients with severe asthma. Allergy 2020; 75(6): 1507– 1510. Wiley Online Library PubMed Web of Science®Google Scholar
171. Antonicelli L, Tontini C, Marchionni A, Lucchetti B, Garritani MS, Bilò MB. Forced oscillation technique as method to document and monitor the efficacy of mepolizumab in treating severe eosinophilic asthma. Allergy 2020; 75(2): 433– 436. Wiley Online Library PubMed Web of Science®Google Scholar
172. Agache I, Akdis C, Akdis M, et al. EAACI biologicals guidelines‐recommendations for severe asthma. Allergy 2020: 1– 31. https://doi.org/10.1111/all.14425 PubMed Web of Science®Google Scholar
173. Lombardi C, Passalacqua G, Bagnasco D. Severe asthma, biologicals, and auto‐ injection: Yes, no, may be! Allergy 2020; 75(2): 444–445. Wiley Online Library PubMed Web of Science®Google Scholar
174. Sokolowska M, Rovati GE, Diamant Z, et al. Current perspective on eicosanoids in asthma and allergic diseases – EAACI Task Force consensus report, part I. Allergy 2020. https://doi.org/10.1111/all.14295 Google Scholar
175. Maun HR, Jackman JK, Choy DF, et al. An allosteric anti‐tryptase antibody for the treatment of mast cell‐mediated severe asthma. Cell 2020; 180(2): 406. CAS PubMed Web of Science®Google Scholar
176. Kere M, Gruzieva O, Ullemar V, et al. Effects of inhaled corticosteroids on DNA methylation in peripheral blood cells in children with asthma. Allergy 2020; 75(3): 688– 691. Wiley Online Library PubMed Web of Science®Google Scholar
177. Schmitt J, Wüstenberg E, Küster D, Mücke V, Serup‐Hansen N, Tesch F. The moderating role of allergy immunotherapy in asthma progression: Results of a population‐based cohort study. Allergy 2020; 75(3): 596– 602. Wiley Online Library CAS PubMed Web of Science® Google Scholar
178.Boonpiyathad T, Pradubpongsa P, Mitthamsiri W, Satitsuksanoa P, Jacquet A, Sangasapaviliya A. Allergen‐specific immunotherapy boosts allergen‐specific IgD production in house dust mite‐ sensitized asthmatic patients. Allergy 2020; 75(6): 1457– 1460. Wiley Online Library CAS PubMed Web of Science® Google Scholar
179. Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: A retrospective database study in France. Allergy 2019; 74(7): 1317– 1326. Wiley Online Library CAS PubMed Web of Science® Google Scholar
180. Wahn U, Bachert C, Heinrich J, Richter H, Zielen S. Real‐world benefits of allergen immunotherapy for birch pollen‐associated allergic rhinitis and asthma. Allergy 2019; 74(3): 594– 604. Wiley Online Library CAS PubMed Web of Science®Google Scholar
181. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long‐term relief in allergic rhinitis and reduces the risk of asthma: A retrospective, real‐world database analysis. Allergy 2018; 73(1): 165– 177. Wiley Online Library CAS PubMed Web of Science®Google Scholar
182. Mösges R, Bachert C, Panzner P, et al. Short course of grass allergen peptides immunotherapy over 3 weeks reduces seasonal symptoms in allergic rhinoconjunctivitis with/without asthma: A randomized, multicenter, double‐blind, placebo‐controlled trial. Allergy 2018; 73(9): 1842– 1850. Wiley Online Library CAS PubMed Web of Science®Google Scholar
183. Asaria M, Dhami S, van Ree R, et al. Health economic analysis of allergen immunotherapy for the management of allergic rhinitis, asthma, food allergy and venom allergy: A systematic overview. Allergy 2018; 73(2): 269– 283. Wiley Online Library CAS PubMed Web of Science®Google Scholar
184. Hesse L, van Ieperen N, Habraken C, et al. Subcutaneous immunotherapy with purified Der p1 and 2 suppresses type 2 immunity in a murine asthma model. Allergy 2018; 73(4): 862– 874. Wiley Online Library CAS PubMed Web of Science®Google Scholar
185. Crossman‐Barnes CJ, Peel A, Fong‐Soe‐ Khioe R, Sach T, Wilson A, Barton G. Economic evidence for nonpharmacological asthma management interventions: a systematic review. Allergy 2018; 73(6): 1182– 1195. Wiley Online Library PubMed Web of Science®Google Scholar
186. Bousquet J, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on Asthma and COVID‐19 (June 2, 2020). Allergy 2020. https://doi.org/ 10.1111/all.14471 Web of Science®Google Scholar
187. Avdeev S, Moiseev S, Brovko M, et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID‐19. Allergy 2020; 75: 2703– 2704. https://doi.org/10.1111/all.14420 Wiley Online Library CAS PubMed Web of Science®Google Scholar
188. Zhang J‐J, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75(7): 1730– 1741. Wiley Online Library CAS PubMed Web of Science®Google Scholar
189. Heffler E, Detoraki A, Contoli M, et al. COVID‐19 in Severe Asthma Network in Italy (SANI) patients: Clinical features, impact of comorbidities and treatments. Allergy 2020. https://doi.org/10.1111 /all.14532 Wiley Online Library Google Scholar
190. Antonicelli L, Tontini C, Manzotti G, et al. Severe asthma in adults does not significantly affect the outcome of COVID‐19 disease: results from the Italian Severe Asthma Registry. Allergy 2020. https://doi.org/10.1111/all.14558 Wiley Online Library Web of Science®Google Scholar
191. Carli G, Cecchi L, Stebbing J, Parronchi P, Farsi A. Is asthma protective against COVID‐19? Allergy 2020. https://doi.org /10.1111/all.14426 Wiley Online Library PubMed Web of Science®Google Scholar
192. Johnston SL. Asthma and COVID‐19: is asthma a risk factor for severe outcomes? Allergy 2020; 75(7): 1543– 1545. Wiley Online Library CAS PubMed Web of Science®Google Scholar
193. Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID‐19. J Allergy Clin Immunol. 2020;146(2):327– 329.e324. Crossref CAS PubMed Web of Science®Google Scholar
194. Lommatzsch M, Stoll P, Virchow JC. COVID‐19 in a patient with severe asthma treated with Omalizumab. Allergy 2020. https://doi.org/10.1111/all.14456 Wiley Online Library PubMed Web of Science®Google Scholar
195. Licari A, Votto M, Brambilla I, et al. Allergy and asthma in children and adolescents during the COVID outbreak: what we know and how we could prevent allergy and asthma flares. Allergy 2020; 75(9): 2402– 2405. Wiley Online Library CAS PubMed Web of Science®Google Scholar
196. Sokolowska M, Lukasik Z, Agache I, et al. Immunology of COVID‐19: mechanisms, clinical outcome, diagnostics and perspectives – a report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy 2020. https://doi.org/10.1111/all.14462 Web of Science® Google Scholar
197. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy 2020; 75(7): 1564– 1581. Wiley Online Library CAS PubMed Web of Science®Google Scholar
198. Riggioni C, Comberiati P, Giovannini M, et al. A compendium answering 150 questions on COVID‐19 and SARS‐CoV‐2. Allergy 2020: 1–39. https://doi.org /10.1111/all.14449 PubMed Web of Science®Google Scholar
199. Radzikowska U, Ding M, Tan GE, et al. Distribution of ACE2, CD147, CD26 and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy 2020: 1– 16. https://doi.org /10.1111/all.14429 PubMed Web of Science® Google Scholar
200. Wang JY, Pawankar R, Tsai HJ, Wu SL, Kuo WS. COVID‐19 and Asthma, the Good or the Bad? Allergy 2020. https://doi.org /10.1111/all.14480 Google Scholar
201. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS‐CoV‐2 and the type I interferon response. PLoS Pathog. 2020; 16(7):e1008737. Crossref CAS PubMed Web of Science®Google Scholar
202. Beaney T, Salman D, Samee T, Mak V. Assessment and management of adults with asthma during the covid‐19 pandemic. BMJ 2020; 369: m2092. PubMed Google Scholar
203. Leonardi A, Fauquert JL, Doan S, et al. Managing ocular allergy in the time of COVID‐19. Allergy 2020; 75(9): 2399– 2402. Wiley Online Library CAS PubMed Web of Science®Google Scholar
204. Vultaggio A, Agache I, Akdis CA, et al. Considerations on Biologicals for Patients with allergic disease in times of the COVID‐19 pandemic: an EAACI Statement. Allergy 2020. https://doi.org/ 10.1111/all.14407 Wiley Online Library Web of Science®Google Scholar
205. Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: Practical considerations on the organization of an allergy clinic – an EAACI/ARIA Position Paper. Allergy 2020. https://doi.org/ 10.1111/all.14453 Wiley Online Library Google Scholar
206. Klimek L, Jutel M, Akdis C, et al. Handling of allergen immunotherapy in the COVID‐19 pandemic: An ARIA‐EAACI statement. Allergy 2020; 75(7): 1546– 1554. Wiley Online Library PubMed Web of Science®Google Scholar
207. Bousquet J, Akdis C, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: An ARIA‐EAACI statement. Allergy 2020. https://doi.org/10.1111/all.14302 Wiley Online Library Web of Science®Google Scholar
208. Klimek L, Hagemann J, Alali A, et al. Telemedicine allows quantitative measuring of olfactory dysfunction in COVID‐19. Allergy 2020. https://doi.org/ 10.1111/all.14467 Web of Science® Google Scholar
209.Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy 2018; 73(5): 1053– 1063. Wiley Online Library CAS PubMed Web of Science®Google Scholar
210.Bilò MB, Pravettoni V, Mauro M, Bonadonna P. Treating venom allergy during COVID‐19 pandemic. Allergy 2020. https://doi.org/10.1111/all.14473 Google Scholar
CREDITS: https://doi.org/10.1111/all.14607