C. Echevarriaa,b, J. Steerb,c, and S. C. Bourke b,c
aDepartment of Respiratory, Royal Victoria Infirmary, Newcastle Upon Tyne, UK; b Translational and Clinical Research Institute, Medical School, Newcastle University, Newcastle Upon Tyne, UK; cDepartment of Respiratory, North Tyneside General Hospital, Newcastle Upon Tyne, UK
Abstract
International Classification of Disease 10 (ICD-10) codes record hospital admissions. We aimed to measure the accuracy of COPD exacerbation (ECOPD) codes and examine coding practices for COPD exacerbation.
Prospective screening and ICD-10 codes were used to identify potential ECOPD within the DECAF internal validation cohort. Two coding searches were performed. The first search identified patients with an ECOPD discharge code, and a second, broad search was developed to identify all clinically confirmed ECOPD.
717 of 1,122 (64%) patients with an ECOPD code had confirmed ECOPD. Common reasons for misclassification in the 405 patients who did not have an ECOPD included: lack of obstructive spirometry to diagnose COPD; and hospital admission due to progressive malignancy, asthma or cardiovascular disease. The broad search identified an additional 297 patients with ECOPD missed by the ECOPD codes. The vast majority of this group had pneumonia complicating ECOPD.
ECOPD codes are insufficiently reliable to identify patients with clinically confirmed ECOPD for the purposes of audit or research. Search strategies should include pneumonia codes, specialist review of medical notes and spirometry confirmation of COPD.
Keywords: COPD, COPD exacerbation, pneumonia, coding, non-invasive ventilation
Background
Healthcare systems use ICD (International Classification of Disease) codes to record hospital admission diagnoses which are, in turn, frequently used to identify populations of patients for audit and research 1–3. A study looking at ICD-9 codes for COPD showed that codes may not agree with COPD definitions used in clinical trials 4. A study of ICD-9 codes in hospitalised patients with COPD showed that the use of respiratory clinicians to review notes, in addition to coding, resulted in a different population to those identified with coding alone, and different mortality outcomes 5. This has potentially serious implications for the reliability of research and audit performed using ICD codes alone. There is a paucity of research looking at the accuracy of ICD-10 codes in COPD patients admitted to hospital.
Codes are complex, with large variations in the population identified depending on the precise codes used 6, and may not accurately reflect a clinical diagnosis of COPD 7. The predominant ICD-10 codes for an exacerbation of COPD begin with “J44” but a number of alternative codes could be used for COPD, such as J43 for emphysema or J40 for bronchitis. The availability of previous confirmatory spirometry is poor in those admitted with ECOPD 8, further limiting diagnostic accuracy. A study of ICD-10 codes in primary care showed that spirometry was only performed in 58% of patients recorded as having COPD 9. In addition, the diagnostic and coding approach to patients with pneumonic exacerbations of COPD (pECOPD) is unclear.
In prognostic research, failure to identify the correct study population results in predictive models with a poor clinical performance in the general population 10. For example, a retrospective study of ECOPD may identify patients using ECOPD codes, but may include patients with heart failure incorrectly coded as ECOPD and miss those that had a pneumonic exacerbation.
Therefore, this study examined ECOPD codes to establish:
1. What proportion of patients with a discharge code of ECOPD had a clinically confirmed diagnosis of ECOPD?
2. How many patients with a clinically confirmed diagnosis of ECOPD were missed by the ECOPD coding search?
Methods
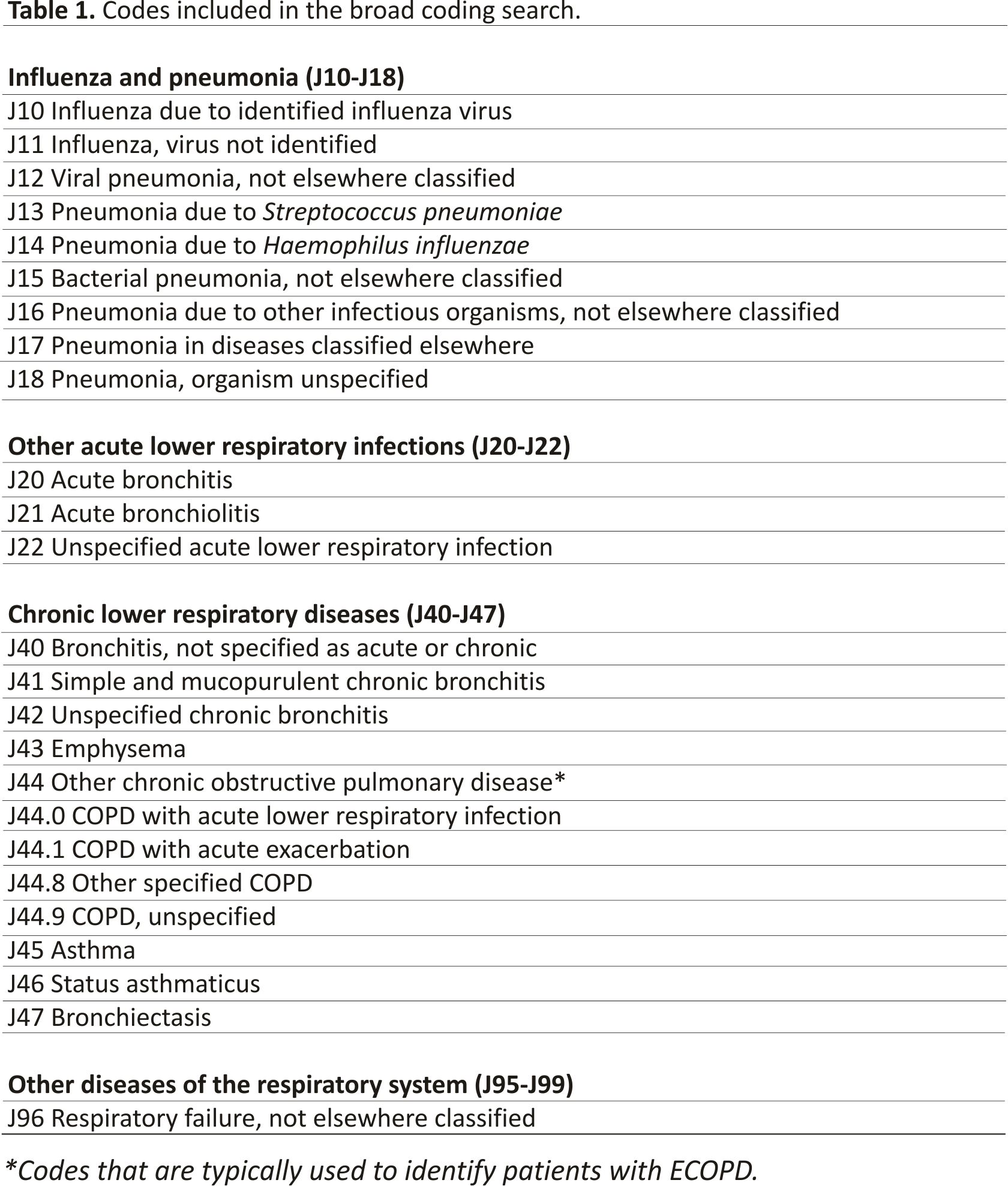
The DECAF internal validation study comprised of two hospitals in the North-East of England with different catchment areas- one urban, one rural. Consecutive admissions of patients admitted to hospital between January 2012 and May 2013 were identified in two searches of hospital discharge codes. The first search included all the J44 ECOPD codes, and the second was an extremely broad coding search as shown in Table 1. The broad coding search was developed in the DECAF derivation study and refined in the internal validation study 11,12 with the aim of identifying all ECOPD without fail. To ensure the broad search did not miss patients with ECOPD, daily screening of all hospital wards was performed for a three month period with case note review of all adults admitted with respiratory symptoms and/or known COPD.
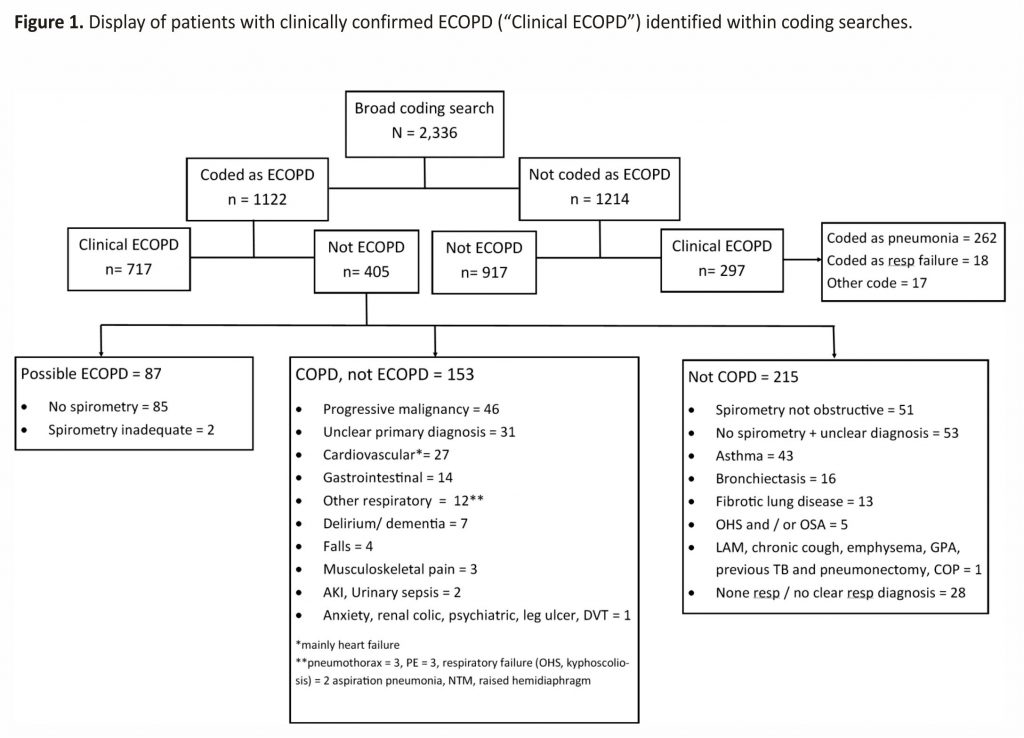
A clinically confirmed diagnosis of COPD required a compatible clinical history and obstructive spirometry, and an exacerbation was defined based on the presence of symptoms as per GOLD guidelines. There was no time limitation for confirmatory spirometry, which could be prior to, during, or after admission. Two respiratory specialists reviewed medical records and patient episodes were labelled by consensus as 1) having a proven clinical diagnosis of an ECOPD with obstructive spirometry and a primary ECOPD diagnosis (a clinically confirmed ECOPD, or “clinical ECOPD” in Figure 1) 13; or 2) unproven ECOPD. Unproven ECOPD included those with a clinical diagnosis of COPD but no confirmatory spirometry from any time (“possible ECOPD”); those with underlying COPD who were admitted to hospital but did not have convincing features of a COPD exacerbation (“COPD, not ECOPD”); and those with no evidence of underlying COPD (“not COPD”).
To minimise missing data, in particular with regards to spirometry, pre-existing COPD diagnoses and other co-morbidities, all medical records were reviewed, including paper and electronic records, and hospital and primary care data sources. For spirometry data, this additionally included searching lung function machines and databases that were not electronically linked with care records.
Results
The J44 ECOPD discharge coding search returned 1,122 patients who were admitted to hospital (entitled “Coded as ECOPD” in Figure 1), of whom 717 (64%) were confirmed as having a clinical diagnosis of an ECOPD. The diagnoses for the 405 patients in this group who were incorrectly coded as an ECOPD are shown in three boxes in Figure 1, labelled “possible ECOPD” (n = 87), “COPD, not ECOPD” (n = 153) and “not COPD” (n = 215). Lack of spirometry/obstructive spirometry or admission due to progressive malignancy, asthma and cardiovascular disease were common reasons for misclassification. In the 87 patients labelled as “possible ECOPD”, presenting symptoms were consistent with an ECOPD and the previous history suggested COPD, but no adequate preadmission or follow-up spirometry was available, despite checking paper and digital records from primary and secondary care. Of interest, in the 1,122 patients coded as an ECOPD, only 138 (12.3%) had missing spirometry.
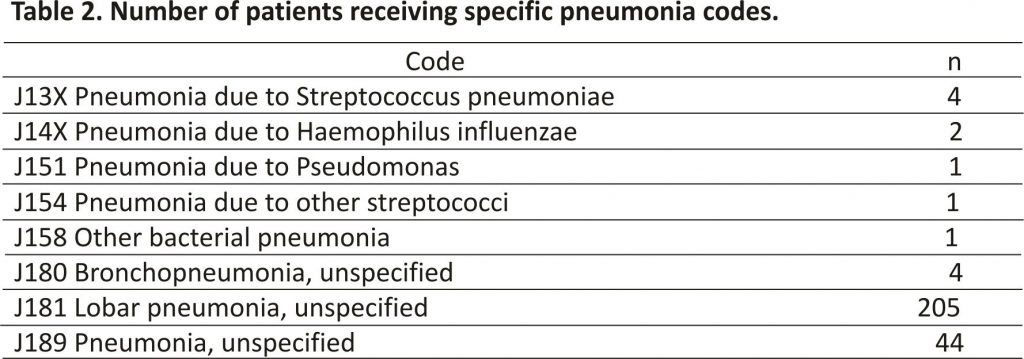
In the broad coding search, 1,014 patients (43%) from a total of 2,336 had a clinical diagnosis of ECOPD (Figure 1). The 1,014 with a clinical ECOPD includes 717 correctly coded as ECOPD (J44), and 297 incorrectly coded who are shown in separate boxes in Figure 1. Therefore, 297 (29%) with a clinical diagnosis ECOPD were missed by the J44 ECOPD coding search. Of these, 262 were coded as pneumonia (see Table 2); 19 as a respiratory failure (J69); 7 as lower respiratory tract infection (J22); 4 as emphysema (J43); 3 as bronchiectasis (J47); and 1 each as upper respiratory tract infection (J39) and influenza (J10). Of the 262 patients with clinically confirmed ECOPD coded as pneumonia, 246 (94%) had an additional (secondary) code that included a J44 ECOPD code.
Discussion
Using discharge J44 ECOPD codes we have shown that only two in three patients have a clinically confirmed diagnosis of ECOPD. Furthermore, these codes will miss almost one in three patients admitted with a clinically confirmed ECOPD, though this can be mitigated by including pneumonia codes as the primary diagnosis within the search to reduce the false-negative rate. Our study has highlighted the inadequacy of using ECOPD ICD-10 codes alone as a strategy to identify populations for research and audit in ECOPD. Our results show that we should question the reliability and generalisability of research studies that use ICD codes alone to identify patients; specialist review of the medical notes is required for diagnostic confidence.
It is important to recognise that J44 ECOPD codes will miss patients with pneumonic exacerbations of COPD. In current clinical practice, community-acquired pneumonia and pAECOPD are not exclusive diagnoses. For example, 23% of patients in the UK CAP audit had co-existent COPD 14, and 18% of patients with AECOPD had co-existent pneumonia in the UK COPD audit 8. It is clear that a consistent approach is required, as variability in the inclusion/exclusion of patients with pneumonic exacerbation of COPD will have an important impact on mortality figures.
The rate of missing data for spirometry in our study amongst those coded as having an ECOPD was only 12.3% which is far lower than in other studies and UK national audit data 8. In our study we made extraordinary efforts to obtain spirometry data, which included checking all secondary care and primary data sources; with several primary care contacts made, and a visit in person if needed. This level of effort is not feasible in usual clinical care and raises the suspicion that the ongoing problem with missing spirometry data in UK audits relates to challenges in accessing spirometry results. Technical solutions are required so that spirometry results from primary or secondary care are available to any clinician attending the patient.
This study has several strengths. The population was well defined, COPD diagnosis was confirmed by respiratory clinicians, consecutive patients were recruited and missing data rates were small. In our coding analysis, survivor bias is not an issue so we included patients that had to follow up spirometry and/or life-limiting diagnoses such as metastatic malignancy. Finally, we performed daily screening of wards to confirm the validity of our broad coding search and very few patients with a clinical diagnosis of ECOPD were not identified through the broad search: as recommended, our search strategy is clearly described, broad and validated 5. The main limitation of our coding data from the internal validation cohort is that it only includes two hospitals. Whilst coding practices will vary between sites, one of the key findings relates to the ICD-10 classification system itself, namely the lack of codes for pECOPD. Furthermore, potential variation in coding practices supports our assertion that ICD codes alone are an unreliable method of identifying generalisable populations for research purposes.
This study focuses on the accuracy of ICD-10 codes in ECOPD, including pECOPD. This is also of clinical relevance, particularly in regard to pECOPD. The usefulness of a search depends upon its function, and there is a trade-off between accuracy and capture rate 15. In prognostic research, initial broad searches are preferential because identifying consecutive patients is crucial to avoid missing patients which biases results: patients are not missed at random 10,16. An example of this is highlighted by Lacasse et al., who showed a significant difference in mortality between groups who were identified using different methods, and caution against the use of administrative databases alone to identify patients 5. In common with our analysis, they also concluded that diagnostic confirmation is required by an appropriate specialist reviewing medical records. Stein et al. looked at four different searches using ICD 9 codes to identify ECOPD, all of which had a negative predicted value above 90%, specificity above 99%, a positive predicted value of between 81 and 97%, and sensitivities between 12 to 25% 7. Although the overall accuracy of some of these searches was low, the capture was broad, unlike Ginde et al. 17 who reported high accuracy rates of ECOPD ICD-9 codes in patients attending the emergency department, with incomplete capture of proven ECOPD. Neither study included pECOPD or the need for spirometric confirmation of COPD.
Conclusions
We have shown that ECOPD ICD-10 codes are unreliable in identifying patients with ECOPD and miss patients with pneumonic ECOPD; for the purposes of audit and research, coding searches should be supported by careful case note review to ensure diagnostic accuracy.
Acknowledgements
The authors thank all those involved in data collection and management in the previous DECAF studies.
Contributors
CE, JS, and SCB conceived, designed, and analysed the study. CE and JS wrote the initial draft of the manuscript, and all authors were involved in re-drafting.
Declaration of interest statement
John Steer has no conflicts of interest to declare. Carlos Echevarria reports grants from National Institute of Health Research, outside of the submitted work. Stephen Bourke reports grants from National Institute of Health Research, Philips Respironics and from Pfizer Open Air, personal fees from Pfizer, AstraZeneca, Chiesi and ResMed, and non-financial support from Boehringer Ingelheim and GlaxoSmithKline outside the submitted work. No author has financial relationships with any organisation that might have an interest in the submitted work.
Ethics approval
Ethics approval was provided by NRES Committee North East, UK.
Data sharing statement
Any individuals or parties interested in accessing data should contact CE or SCB.
Additional information
Funding
Data was used from the DECAF studies which were funded by the Department of Health, Breathe North appeal and Novartis Pharmaceuticals UK, and supported by the NIHR Clinical Research Network. The salary of CE was funded by the Northumbria NHS Foundation Trust Teaching and Research Fellowship programme.
References
1. Shorr AF, Sun X, Johannes RS, et al. Validation of a novel risk score for severity of illness in acute exacerbations of COPD. Chest. 2011; 140(5):1177–1183. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
2. Hodgson LE, Dimitrov BD, Congleton J, et al. A validation of the National Early Warning Score to predict outcome in patients with COPD exacerbation. Thorax. 2017; 72(1): 23–30. DOI:10.1136/thoraxjnl-2016-208436 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
3. Edwards L, Perrin K, Wijesinghe M, et al. The value of the CRB65 score to predict mortality in exacerbations of COPD requiring hospital admission. Respirology. 2011; 16(4):625–629. DOI:10.1111/j.1440-1843.2011. 01926.x [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
4. Prieto-Centurion V, Rolle AJ, Au DH, CONCERT Consortium, et al. Multicenter study comparing case definitions used to identify patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(9):989–995. DOI:10. 1164/rccm.201406-1166OC [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
5. Lacasse Y, Daigle JM, Martin S, et al. Validity of chronic obstructive pulmonary disease diagnoses in a large administrative database. Can Respir J. 2012; 19(2):e5–e9. DOI: 10.1155/2012/260374 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. Copd. 2010; 7(3):164 –171. DOI:10.3109/15412555.2010. 481696 [Taylor & Francis Online], [Google Scholar]
7. Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012; 141 (1):87–93. DOI:10.1378/chest.11-0024 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
8. Stone RA, Holzhauer-Barrie J, Lowe D, et al. COPD: Who cares matters. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Clinical Audit of COPD Exacerbations Admitted to Acute Units in England and Wales. 2014; 2015 [Google Scholar]
9. Schneider A, Gantner L, Maag I, et al. Are ICD-10 codes appropriate for performance assessment in asthma and COPD in general practice? Results of a cross-sectional observational study. BMC Health Serv Res. 2005; 5(1):11 DOI:10. 1186/1472-6963-5-11 [Crossref], [PubMed], [Google Scholar]
10. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015; 162(1): W1–73. DOI:10.7326 /M14-0698 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
11. Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012; 67(11):970–976. DOI:10.1136/thoraxjnl-2012-202103 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
12. Echevarria C, Steer J, Heslop-Marshall K, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016; 71(2):133 – 140. DOI:10.1136/thoraxjnl-2015-207775 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
13. From the Global Strategy for the Diagnosis Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from: http://goldcopd. org [Google Scholar]
14. Daniel P, Bewick T, Welham S, et al. Adults miscoded and misdiagnosed as having pneumonia: results from the British Thoracic Society pneumonia audit. Thorax. 2017; 72(4):376– 379. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
15. Lee TM, Tu K, Wing LL, et al. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017; 27(1):34 DOI:10.1038/s41533-017-0035-9 [Crossref], [PubMed], [Google Scholar]
16. Royston P, Moons KG, Altman DG, et al. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
17. Ginde AA, Tsai CL, Blanc PG, et al. Positive predictive value of ICD-9-CM codes to detect acute exacerbation of COPD in the emergency department. Jt Comm J Qual Patient Saf. 2008;34(11): 678–680., Jr. [Crossref], [PubMed], [Google Scholar]
Credits: C. Echevarria, J. Steer & S. C. Bourke (2020) Coding of COPD Exacerbations and the Implications on Clinical Practice, Audit and Research, COPD: Journal of Chronic Obstructive Pulmonary Disease, 17:6, 706-710, DOI: 10.1080/15412 555.2020. 1841745. To link to this article: https://doi.org/10.1080/15412555.2020.1841745