Joseph F. Panarelli MD 1, Marlene R. Moster MD 2, Julian Garcia-Feijoo MD, PhD 3, Brian E. Flowers MD 4, N. Douglas Baker MD 5, Howard S. Barnebey MD 6, Davinder S. Grover MD, MPH 7, Anup K. Khatana MD 8, Bonny Lee MD 1, Tuan Nguyen PhD 9, Michael C. Stiles MD 10, Omar Sadruddin MD, MBA 9, Peng T. Khaw PhD, FRCP 11, 12 INN005 Study Group∗
Purpose
To compare the effectiveness and safety of the MicroShunt (Santen Inc) versus trabeculectomy in patients with primary open-angle glaucoma (POAG).
Design
The prospective, randomized, multi-centre trial was conducted in the United States and Europe.
Participants
Adult patients (aged 40–85 years) with mild to severe POAG inadequately controlled on maximum tolerated medical therapy and intraocular pressure (IOP) ≥ 15 mmHg and ≤ 40 mmHg.
Methods
Patients were randomized 3:1 to stand-alone MicroShunt implantation (n = 395) or trabeculectomy (n = 132), both augmented with mitomycin C (MMC) 0.2 mg/ml for 2 minutes.
Main Outcome Measures
The primary effectiveness endpoint was surgical success, defined as ≥ 20% reduction in mean diurnal IOP from baseline with no increase in glaucoma medications. Secondary endpoints included changes in mean IOP and medication use from baseline and the need for post-operative interventions.
Results
At 2 years, the rate of surgical success was lower in the MicroShunt group than in the trabeculectomy group (50.6% vs. 64.4%, P = 0.005). Mean diurnal IOP was reduced from 21.1 ± 4.9 mmHg at baseline to 13.9 ± 3.9 mmHg at 24 months in the MicroShunt group and from 21.1 ± 5.0 mmHg at baseline to 10.7 ± 3.7 mmHg at 24 months in the trabeculectomy group (P < 0.001 compared with baseline in both groups). Mean medication use decreased from 3.1 to 0.9 in the MicroShunt group and from 2.9 to 0.4 in the trabeculectomy group (P < 0.001 compared with baseline in both groups). Adverse events at 2 years were generally similar in the 2 groups, except that hypotony was more common in eyes undergoing trabeculectomy (51.1% vs. 30.9%, P < 0.001). Repositioning or explantation of the implant occurred in 6.8% of MicroShunt patients. The majority of these patients had device removal at the time of subsequent glaucoma surgery. Vision-threatening complications were uncommon in both groups.
Conclusion
At 2 years, both the MicroShunt and trabeculectomy provided significant reductions in IOP and medication use, with trabeculectomy continuing to have greater surgical success.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords
Antifibrotic agents, Aqueous drainage devices Minimally invasive glaucoma surgery Open-angle glaucoma Trabeculectomy
Abbreviations and Acronyms
BCVA, best-corrected visual acuity, CI, confidence interval, IOP, intraocular pressure, MIGS, micro-invasive glaucoma surgery, MMC, mitomycin C, POAG, primary open-angle glaucoma
Since its description in 1968, the Cairns-type trabeculectomy has been the clinical standard for surgical reduction of intraocular pressure (IOP) in eyes with glaucoma.1 Based on its efficacy, trabeculectomy is the preferred procedure in eyes with advanced glaucoma or very low interventional IOP.2,3,4,5 However, its safety profile —with potentially sight-threatening complications, including bleb leaks, choroidal effusions, hypotony maculopathy, and blebitis/endoph-thalmitis—may limit its value in eyes with more modest therapeutic goals.4,6 Trabeculectomy also requires a high level of surgeon skill and experience to appropriately manage a delicate series of operative and postoperative manipulations and adjustments. The advent of a family of procedures collectively referred to as “micro-invasive glaucoma surgery” (MIGS) has expanded the indications for surgical intervention in eyes with glaucoma. The MIGS family includes procedures that shunt aqueous humour across the trabecular meshwork to Schlemm’s canal or the suprachoroidal space. Some of these procedures require the implantation of a permanent device, whereas others are limited to the incision or excision of various ocular tissues.7,8,9,10,11 In general, MIGS procedures have a more favourable safety profile compared with trabeculectomy, but with lower efficacy. Thus, canal-based MIGS procedures may be viable options for patients who would benefit from surgical IOP reduction but whose more modest therapeutic goals may not justify the risks of trabeculectomy.7, 8, 9, 10, 11
The MicroShunt (Santen Inc) is an implantable device, measuring 8.5 mm in length and 0.35 mm in width, with a lumen diameter of 70 μm and a pair of 0.375-mm fins for intrascleral fixation. The tube length and lumen diameter were selected to accommodate sloughed endothelial cells (40–50 μm) while providing sufficient resistance to limit pressure reduction and minimize the risk of hypotony.12 The MicroShunt is implanted subconjunctivally via an ab-externo approach and provides filtration from the anterior chamber to the subconjunctival space without the need for scleral flap formation, sclerotomy, or iridectomy.
This 2-year trial compared the effectiveness and safety of the MicroShunt with trabeculectomy in patients with uncontrolled primary open-angle glaucoma (POAG), using maximal tolerated medical therapy, in the United States and Europe. Interim 1-year results of this trial have been reported.13 The present study describes the 2-year results of this trial.
Methods
This was a prospective, single-masked, randomized, multicenter, noninferiority trial conducted at 24 sites in the United States and 5 sites in Europe. The study was conducted per the tenets of the Declaration of Helsinki, as well as all applicable local regulations.14 The study protocol was approved by all relevant ethics boards, and all subjects provided written informed consent to participate. The trial was registered at ClinicalTrials.gov (NCT0188142 5).
The study design, eligibility criteria, treatments, and surgical details have been reported previously.13 Briefly, this trial enrolled patients aged 40 to 85 years with mild to severe POAG (visual field mean deviation –3.0 decibels or worse) with IOP ≥ 15 mmHg and ≤ 40 mmHg, inadequately controlled on maximally tolerated medical therapy. Key exclusion criteria included secondary glaucoma, vision level of no light perception, prior ocular surgery involving the conjunctiva, and the requirement for general anaesthesia for the study procedure. Only 1 eye was enrolled per patient. Eligible subjects were randomly assigned 3:1 to undergo MicroShunt implantation or trabeculectomy; unequal allocation was deemed applicable to this confirmatory trial because it allowed the recruitment of a sufficient number of eyes in the treatment arm to detect rare adverse events. Both procedures were augmented with mitomycin C (MMC) 0.2 mg/ml applied for 2 minutes via saturated sponges on bare sclera. For the MicroShunt procedure, a 1-mm double-step knife was used to fashion a narrow scleral track with a wider scleral pocket at its distal end. The device was threaded through the track and the fins tucked into the pocket. The flow of aqueous humour from the anterior chamber was confirmed, and Tenon’s capsule and conjunctiva were sutured in watertight closure. Fornix-based trabeculectomy was performed in standard fashion with watertight closure of Tenon’s capsule and conjunctiva.
Patients were evaluated at 1 day, 1 and 4 weeks, and 3, 6, 12, 18, and 24 months after surgery. Parameters measured included best-corrected visual acuity (BCVA), IOP by Goldmann tonometry (mean of 2 readings within 2 mmHg or 3 readings if the difference was > 2 mmHg), medication use, and complications. The trial protocol specified that the window for year 1 (12-month) visits was on study days 330 to 420 and the window for year 2 (24-month) visits was on study days 690 to 780. At baseline and months 12 and 24, diurnal IOP was assessed at 9 am, 12 pm, and 4 pm. Before randomization, an interventional IOP (above which an IOP-lowering intervention would be applied) was set for each eye as either 21 mmHg or preoperative IOP if it was < 21 mmHg or as an IOP selected by the investigator based on individual risk factors. Interventions included laser suture lysis, needling, and laser disruption of outflow obstruction; medications were generally initiated only after unsuccessful attempts at these procedural interventions unless such attempts were believed unlikely to achieve interventional IOP.
Efficacy was analyzed in the intent-to-treat population (all randomized subjects) with the baseline-observation-carried-forward approach to the imputation of missing data for the primary effectiveness endpoint, defined as a ≥ 20% reduction from baseline in mean diurnal IOP at the month 24 visit without increasing the number of preoperative IOP-lowering medications, consistent with guidelines set by the World Glaucoma Association.15 Endpoint failures included loss of light perception at 2 consecutive visits, IOP < 6 mmHg at 2 consecutive visits after month 3, incisional or laser reoperation (except for suture lysis, needling, or laser removal of outflow obstruction) for complications or inadequate IOP control, and the need for oral carbonic anhydrase inhibitors for IOP control. A Farrington-Manning test was used to construct 95% confidence intervals (CIs) around the difference in success rates between treatment groups. Farrington-Manning tests were performed to test for non-inferiority, and Wald-type tests were performed to test the equality of rates. Time to first failure was assessed using Kaplan– Meier survival analysis with treatments compared using the log-rank test. Prespecified subgroup analyses in patients with baseline mean diurnal IOP < 18, 18 to < 21, and ≥ 21 mmHg were conducted.
Secondary effectiveness endpoints included mean diurnal IOP change at month 24 and the need for postoperative interventions by month 24. Mean diurnal IOP change was evaluated using a mixed-effects model for repeated measures with Hochberg step-up adjusted P values based on a 2.5 mmHg noninferiority margin (as in the Tube Versus Trabeculectomy study).16 The mean number of glaucoma medications required at each visit was an exploratory efficacy endpoint.
The proportions of eyes in each group requiring interventions through month 24 and the incidence of specific qualifying reoperations (bleb revision, repositioning or removal of implant, resuturing scleral flap, trabeculectomy, glaucoma drainage device, and iridotomy/iridectomy) were compared using Wald-type 2-sample proportions tests for the equality of rates.
Safety was analyzed in the safety population, consisting of all subjects who underwent surgery. Prespecified safety endpoints included the occurrence of adverse events, development of cataracts as assessed using the Lens Opacities Classification System, Version III in phakic eyes, time for postoperative BCVA to return to baseline, and change in endothelial cell density. Endothelial cell density was measured using a Konan specular microscope, with all measurements performed at the endothelial cell density Corneal Image Analysis Reading Center at the Department of Ophthalmology and Visual Sciences at Case Western Reserve University School of Medicine.13
Sample size calculations were based on a Z-test with normal distribution approximation and assuming an annual dropout rate of 6%.13 A sample size of 514 patients was calculated for the primary effectiveness endpoint assuming a month 12 IOP success rate of 0.74, a 1-sided significance level of 0.025, 90% statistical power, and a noninferiority margin of 0.15 (15%).
Results
Subject Disposition and Demographics
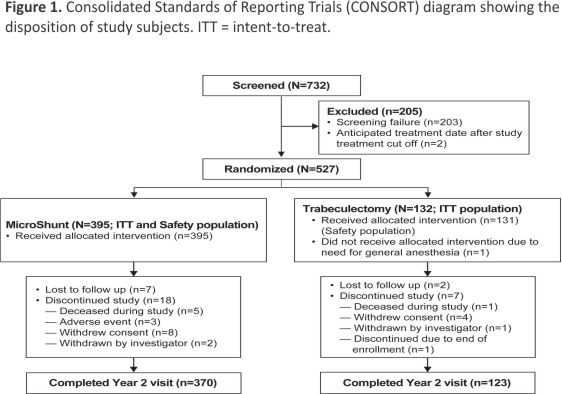
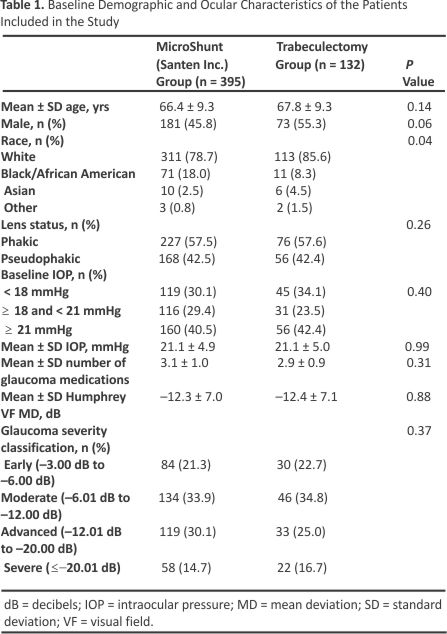
The intent-to-treat population included 527 subjects who were randomized to treatment with the MicroShunt (n = 395) or trabeculectomy (n = 132) (Fig 1). Of these, all but 1 patient randomized to trabeculectomy (who required general anaesthesia and was thus ineligible) underwent the assigned study procedure and comprised the safety population. By the end of the second year of the study, 9 subjects (7 in the MicroShunt and 2 in the trabeculectomy group) were lost to follow-up, and 25 subjects (18 in the MicroShunt group and 7 in the trabeculectomy group) discontinued the study. Of the 18 patients in the MicroShunt group who discontinued, 5 died, 3 discontinued due to adverse events, 8 withdrew consent, and 2 were withdrawn by the investigator. Of the 7 patients in the trabeculectomy group who discontinued, 1 died, 4 withdrew consent, 1 was withdrawn by the investigator, and 1 had been enrolled after closure of the study enrollment window. Small proportions of patients visited at times outside the prespecified follow-up times. Specifically, of the 527 randomized patients, only 11 (2.1%) visited outside the 12-month window of 330 to 420 days and only 8 (1.5%) visited outside the 24-month window of 690 to 780 days. The demographic and ocular characteristics of the study population are shown in Table 1. Baseline characteristics were similar in the 2 groups except for ethnicity, with a higher proportion of Black subjects in the MicroShunt group than in the trabeculectomy group (18.0% vs. 8.3%, P = 0.04).
Primary Effectiveness End Point
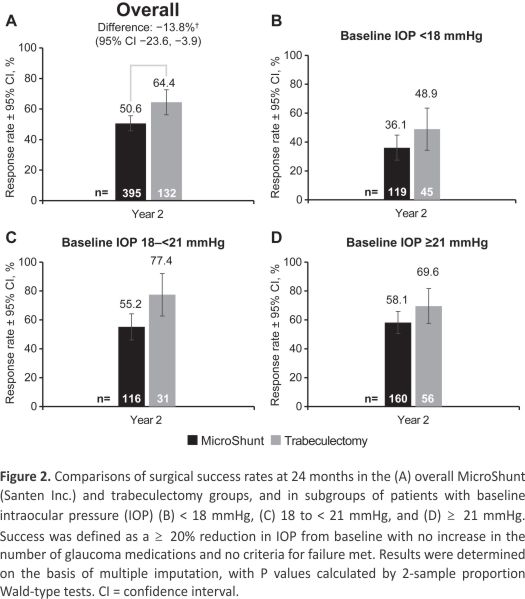
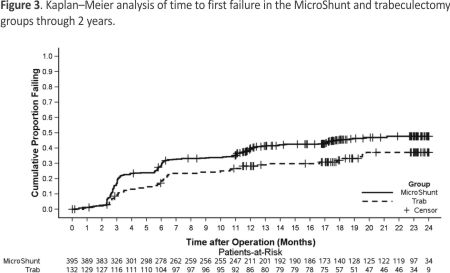
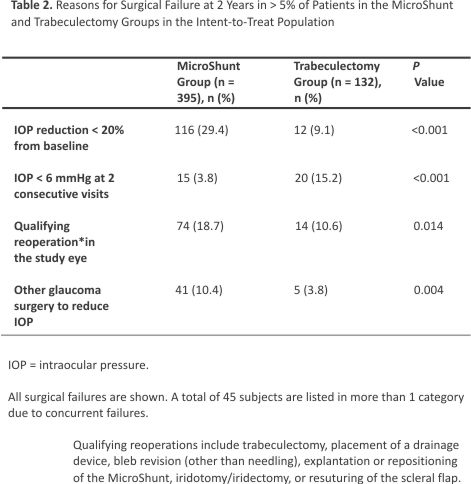
Surgical success (≥ 20% reduction in IOP from baseline with no increase in the number of glaucoma medications) was observed in 50.6% of subjects in the MicroShunt group and 64.4% in the trabeculectomy group (P = 0.005) (Fig 2A). Sub- group analysis showed that at year 2, the between-group difference in surgical success in eyes with baseline IOP < 18 mmHg was –12.8% (95% CI, –29.5 to 4.0; P = 0.141; Fig 2B); in eyes with IOP ≥ 18 mmHg but < 21 mmHg, this difference was –22.2% (95% CI, –41.7 to –2.8, P = 0.012; Fig 2C); and in eyes with IOP ≥ 21 mmHg, this difference was –11.5% (95% CI, –26.4 to 3.3; P = 0.114; Fig 2D). The time to first failure in each group is shown in Figure 3. Median survival times could not be determined, because less than 50% of the eyes in each group had failed by the study’s end. The nature of failures is shown in Table 2. Failure to achieve an IOP reduction of ≥ 20% from baseline was the most common reason for failure in the MicroShunt group, whereas persistent hypotony was the leading cause of failure in the trabeculectomy group.
Figure 2. Comparisons of surgical success rates at 24 months in the (A) overall MicroShunt (Santen Inc.) and trabeculectomy groups, and subgroups of patients with baseline intraocular pressure (IOP) (B) < 18 mmHg, (C) 18 to < 21 mmHg, and (D) ≥ 21 mmHg. Success was defined as a ≥ 20% reduction in IOP from baseline with no increase in the number of glaucoma medications and no criteria for failure met. Results were determined based on multiple imputations, with P values calculated by 2-sample proportion Wald-type tests. CI = confidence interval.
Secondary Effectiveness End Points
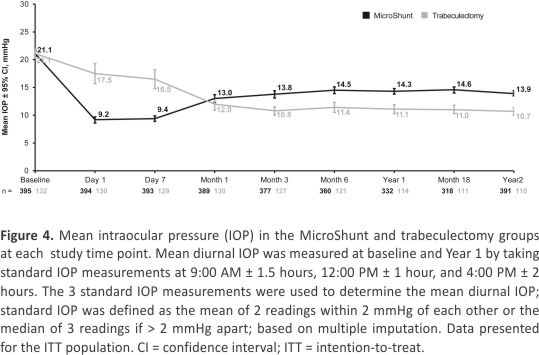
The mean IOP by treatment group at each study time point is shown in Figure 4. Mean IOP was higher in eyes in the trabeculectomy compared with the MicroShunt group during the first postoperative week, indicative of tight closure of the scleral flap and subsequent suture lysis after day 7. From month 3 onward, mean IOP was lower in the trabeculectomy group, by approximately 3 mmHg, at each time point through the end of the study. Mean IOP from month 3 to year 2 was approximately 14 mmHg in the eyes in the MicroShunt group and approximately 11 mmHg in the eyes in the trabeculectomy group.
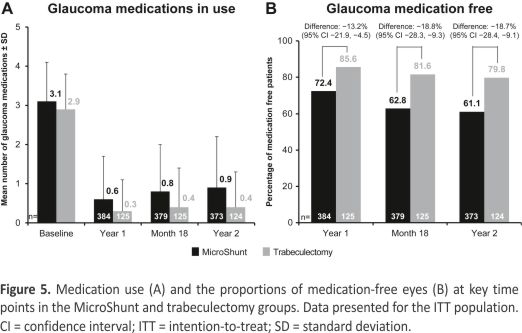
Glaucoma medication use at baseline and months 12, 18, and 24 is shown in Figure 5A. Mean medication use was reduced from 3.1 ± 1.0 at baseline to 0.9 ± 1.3 at month 24 in MicroShunt-treated eyes and from 2.9 ± 0.9 to 0.4 ± 0.9 in trabeculectomy-treated eyes (P < 0.001 compared with baseline in both groups). The proportions of eyes that were medication-free at 12, 18, and 24 months are shown in Figure 5B. At month 24, 61.1% of eyes in the MicroShunt group and 79.8% of eyes in the trabeculectomy group were medication-free.
To assess regional differences, outcomes were compared in the United States and the European Union. The baseline demographic and clinical characteristics were well-matched across groups by location and intervention (Table S3, available at www.aaojournal.org). Surgical success rates were higher in the European Union than in the United States (Fig S6, available at www.aaojournal.org). Patients in the United States who underwent MicroShunt implantation and trabeculectomy had mean diurnal IOP of 21.1 ± 5.1 mmHg and 21.2 ± 5.2 mmHg, respectively, at baseline, decreasing to 14.1 ± 4.1 mmHg and 10.6 ± 3.7 mmHg, respectively, at 2 years (P < 0.001 compared with baseline in both groups). The differences in mean changes from baseline were –6.4 ± 5.8 mmHg in the MicroShunt group and –10.1 ± 5.5 mmHg in the trabeculectomy group, with the between-group difference being statistically significant (P < 0.001).
In comparison, patients in the European Union who underwent MicroShunt implantation and trabeculectomy had mean diurnal IOP of 20.6 ± 3.4 mmHg and 20.3 ± 3.7 mmHg, respectively, at baseline, decreasing to 13.3 ± 2.8 mmHg and 11.5 ± 3.7 mmHg, respectively, at 2 years (P < 0.001 compared with baseline in both groups). The differences in mean changes from baseline were –7.1 ± 3.6 mmHg in the MicroShunt group and –8.3 ± 5.4 mmHg in the trabeculectomy group, with the between-group difference not being statistically significant (P = 0.296). Within the United States, the percentages of medication-free patients were 60.8% in the MicroShunt group and 81.5% in the trabeculectomy group. In the European Union, the corresponding figures were 63.2% and 68.8%, respectively.
Patients with low baseline IOP (< 18 mmHg) were compared in the MicroShunt (n = 119) and trabeculectomy (n = 45) groups. The demographic and clinical characteristics of these 2 groups were similar (Table 4). Mean diurnal IOP ± standard deviation in the MicroShunt and trabeculectomy groups were 16.4 ± 0.9 mmHg and 16.6 ± 0.9 mmHg, respectively, at baseline, decreasing to 12.9 ± 3.2 mmHg and 10.6 ± 4.3 mmHg, respectively, at 2 years (P < 0.001 compared with baseline in both groups), with 66.1% and 69.8%, respectively, being medication-free at 2 years. The composite primary end point of surgical success was achieved by 36.1% of patients in the MicroShunt group and 48.9% in the trabeculectomy group (non-inferiority P = 0.398). Rates of postoperative interventions and postoperative adverse events did not differ significantly in these 2 groups.
Safety
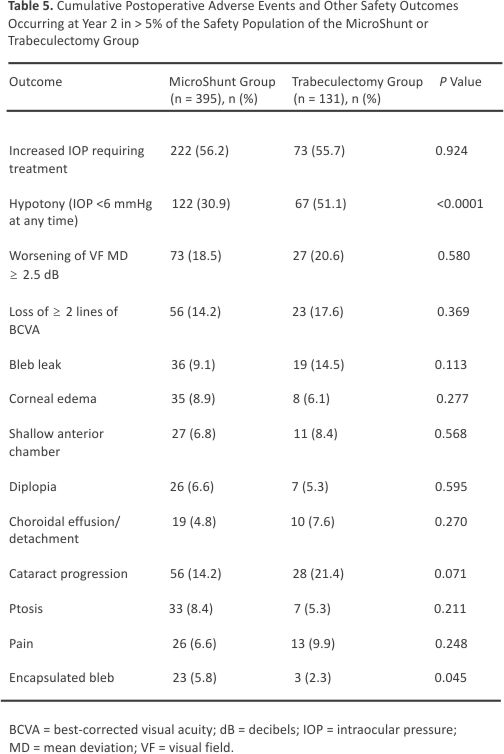
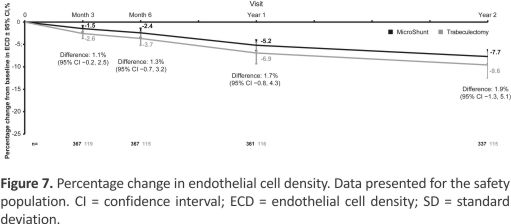
Table 5 shows the frequency and nature of adverse events, each of which occurred in > 5% of subjects in either group through 2 years. The most common adverse event in both the MicroShunt and trabeculectomy groups was increased IOP requiring treatment (56.2% vs. 55.7%; P = 0.924). Hypotony, defined as IOP < 6 mmHg at any time point, was significantly more frequent in the trabeculectomy group than in the MicroShunt group (51.1% vs. 30.9%; P < 0.001). Surgical failure due to hypotony (IOP < 6 mmHg at 2 consecutive visits) also occurred more often in the trabeculectomy group (15.2% vs. 3.8% P < 0.001). Hypotony maculopathy was detected in 0.5% of MicroShunt-treated and 1.5% of trabeculectomy-treated eyes. Endothelial cell density decreased significantly in both groups over 2 years (P < 0.001), with mean reductions of 7.7% in the MicroShunt group and 9.6% in the trabeculectomy group (P = 0.242) (Fig 7). No cases of endophthalmitis occurred in either group.
Mean BCVA was modestly reduced (by ∼5 ETDRS letters) at month 1 in both groups and returned to preoperative values (within 1–3 letters) by month 3 in both groups. Rates of lens opacity (Lens Opacities Classification System, Version III) in phakic eyes at 2 years, defined as postoperative lens opacity or worsening of preexisting lens opacity, were similar in the MicroShunt (41.1% [78/190]) and trabeculectomy (47.6% [30/63]) groups (P = 0.361). Cataract surgery through month 24 was performed in 7.6% of eyes in the MicroShunt group and 13.0% of eyes in the trabeculectomy group (P = 0.095).
Through 2 years of follow-up, a higher percentage of MicroShunt-treated patients than trabeculectomy-treated patients required bleb needling with an antifibrotic agent (most commonly MMC) (24.8% vs. 9.1%; P < 0.001). Of these eyes that underwent needling, 21.4% and 25% from the MicroShunt and trabeculectomy groups, respectively, were needed more than once.
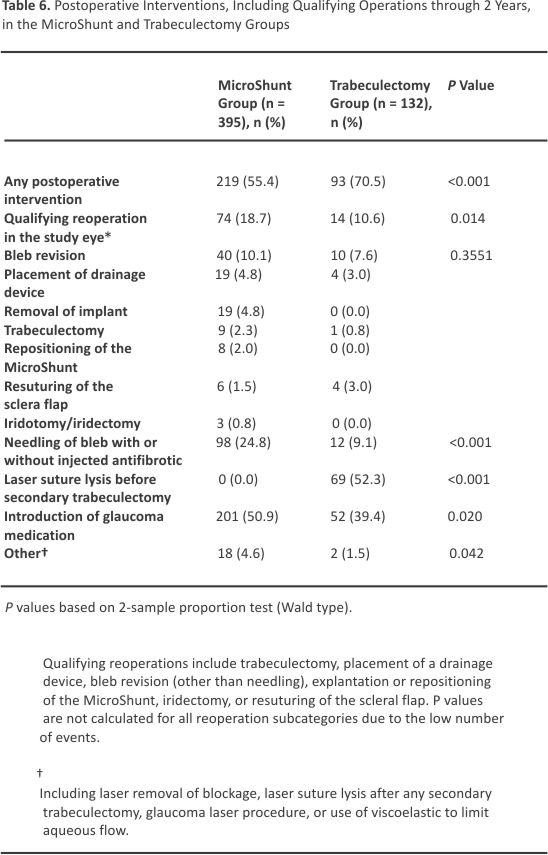
Qualifying reoperations for complications or inadequate IOP control (Table 6) were more common in the MicroShunt group than in the trabeculectomy group (18.7% vs. 10.6%; P = 0.014). Bleb revision with the opening of the conjunctiva was the most common procedure in both groups (10.1% vs. 7.6%). Nineteen patients (4.8%) required MicroShunt explantation, including 4 for device-related reasons/ complications. Of these 4 patients, 2 experienced anterior migration of the implant, requiring device removal due to endothelial concerns; 1 underwent explantation due to persistent hypotony, and 1 underwent explantation due to device-associated erosion of the conjunctiva. The other 15 patients underwent MicroShunt removal at the time of subsequent glaucoma surgery. Eight additional patients (2.0%) required MicroShunt repositioning at the time of open revision.
Discussion
The results of this 2-year prospective, randomized trial comparing the effectiveness and safety of the MicroShunt versus trabeculectomy in eyes with medically uncontrolled POAG showed that IOP reduction and surgical success rates were statistically greater in the trabeculectomy group, similar to the results observed after 1 year.13 The rates of surgical success, defined as a ≥ 20% reduction in baseline IOP without an increase in the number of glaucoma medications, at 2 years were 64.4% in the trabeculectomy group (72.7% at year 1) and 50.6% (53.9% at year 1) in the MicroShunt group. These rates are generally consistent with studies reporting the outcomes of these 2 procedures individually.17, 18,19,20,21,22,23,24 Several retrospective, multicenter studies have reported that the 1-year overall success rates of the MicroShunt ranged from 59% to 74%, depending on the definition of success.17,18,19 A prospective multicenter study reported that the 1-year overall success rate of the MicroShunt was 78%, 20 whereas retrospective single-centre studies have reported overall success rates (with or without medication) of 79% at both 1 year and 2 years.21 The success rates of trabeculectomy at 1 and 3 years were 87% and 79%, in the Tube Versus Trabeculectomy respectively, trial, 22,23 and 92% and 67%, in the Primary Tube Versus Trabeculectomy trial.4,24 A post hoc analysis of the data from the present study stratified patients into tertiles based on baseline IOP. The greatest difference in surgical success between the MicroShunt and trabeculectomy groups was found in patients with a baseline pressure between 18 and 21 mmHg (Fig 2). Patients with a low baseline IOP (< 18 mmHg) were least likely to achieve surgical success in either group, with only 36.1% of patients in the MicroShunt group and 48.9% of patients in the trabeculectomy group meeting the primary endpoint. The inability to safely titrate the IOP to a low number with either procedure may have contributed to the relatively high rate of failure with both procedures in this subset of patients.
A variety of factors account for differences in surgical success when comparing studies, including but not limited to the definition of success, patient demographics and characteristics, surgeon experience and skill, and fixed parameters of the procedure itself. When performing micro-shunting procedures, pharmacologic inhibition of post-operative fibrosis is critical to achieving and maintaining optimal IOP lowering,8 because the device dimensions limit flow and there are no other ways to manipulate bleb formation. Antifibrosis therapy with MMC or 5-fluorouracil is often used both intraoperatively and postoperatively to minimize scarring. The specific antimetabolite used, as well as its dose and method of delivery, is standardized within studies but differs between studies.17, 18, 19, 20, 21, 22 In the current pivotal trial, 0.2 mg/ml of MMC was applied to the scleral bed for 2 minutes, according to a protocol finalized in 2017. Subsequently, the results of Schlenker et al25 have suggested that for MicroShunt implantation, an MMC concentration of 0.2 mg/ml is associated with a higher risk of surgical failure compared with a concentration of 0.4 to 0.5 mg/ml (hazard ratio, 2.51; 95% CI, 1.01–6.23). Likewise, a 2-year, nonrandomized, multicenter study of the MicroShunt device showed that 90.3% of patients treated with MMC 0.4 mg/ml were medication-free, compared with 51.9% of patients treated with MMC 0.2 mg/ml.20 It is possible that outcomes in the MicroShunt arm of the current study would have been improved had a higher dose of MMC been used, especially in patients at higher risk of failure due to scarring. Interestingly, the rate of surgical success with the MicroShunt was substantially different between the United States (47.5%) and Europe (69%), whereas the surgical technique and MMC concentration and delivery were the same. The significantly higher percentage of Black patients in the US cohort may have contributed to this difference because Black race is known to be a risk factor for worse outcomes after glaucoma surgery.26,27
The most common reason for surgical failure through 2 years of follow-up in trabeculectomy-treated eyes was persistent hypotony (15.2% vs. 3.8% in MicroShunt-treated eyes), defined as IOP < 6 mmHg at 2 consecutive visits after 3 months. In contrast, the most common cause of surgical failure in MicroShunt-treated eyes was an IOP reduction of less than 20% from baseline (29.4% vs. 9.1% in trabeculectomy-treated eyes). These data provide evidence of the relative protection against hypotony afforded by the inherent resistance to fluid flow of the MicroShunt device. Conversely, limitations imposed by this inherent resistance may predispose to episcleral fibrosis and IOP elevation, particularly in patients at higher risk for postoperative scarring. Postoperative needling at the slit lamp was performed in 98 patients (24.8%) in the MicroShunt group and 12 patients (9.1%) in the trabeculectomy group (P < 0.001). When performing needle revision, surgeons were allowed to apply additional MMC at their desired dose. Of patients who underwent needling without prior glaucoma reoperation, 25.3% (24/95) of patients in the MicroShunt arm and 54.5% (6/11) of patients in the trabeculectomy arm went on to achieve surgical success. Likewise, open bleb revision in the operating room allowed for surgeons to place additional MMC at the time of the procedure. Forty patients (10.1%) underwent open revision in the operating room in the MicroShunt group, and 10 patients (7.6%) required revision in the trabeculectomy arm. Among patients who underwent bleb revision without prior reoperation, 33.3% (13/39) in the MicroShunt group and 0% (0/10) in the trabeculectomy group (P = 0.045) went on to need traditional glaucoma surgery, including placement of a glaucoma drainage device or trabeculectomy by the end of year 2.
When selecting therapy, the risks of each treatment should be weighed against patient-specific treatment goals. A procedure that carries a higher risk of serious complications such as hypotony may be preferred for patients with more advanced, sight-threatening glaucoma or those at higher risk of surgical failure due to post-operative scarring if this procedure is more likely to result in lower interventional IOP. Conversely, a safer procedure may be better for patients with more modest pressure goals and those at lower risk for filtration failure. The nature and rates of adverse events at 2 years were similar in the 2 groups, except for hypotony, which was more common in eyes undergoing trabeculectomy compared with MicroShunt implantation (51.1% vs. 30.9%, P < 0.001). Adverse events, such as a shallow anterior chamber and choroidal effusions, were transient and generally resolved with conservative management. Only 1 eye in the trabeculectomy group required drainage of choroidal effusions. At year 2, cataract progression was observed in 14.2% of eyes in the MicroShunt group and 21.4% of eyes in the trabeculectomy group (P = 0.071). No serious bleb-related complications, such as blebitis or bleb-related endophthalmitis, were observed in either group.
The skill and experience of the surgeon have a significant effect on the outcome obtained with any surgical procedure. This is particularly true for a procedure such as trabeculectomy that requires a delicate series of operative and postoperative manipulations and adjustments to achieve a positive result. Although trabeculectomy is still regarded as the standard first-line treatment for patients requiring glaucoma surgery, the number of trabeculectomies performed each year continues to decrease while the number of micro-invasive glaucoma surgeries increases.28 Risks of the procedure as well as intraoperative and postoperative challenges are typically cited as the main reasons surgeons are moving away from trabeculectomy. The trabeculectomy arm of the present study demonstrated exceptional outcomes for IOP reduction: The mean IOP at 2 years was 10.7 mmHg, using an average of 0.4 medications. Additionally, few serious complications were observed. All surgeons who participated in this trial had extensive experience with trabeculectomy. The benefits of micro-shunt procedures may include a reduction in the number, variability, and complexity of surgical manipulations, as well as a reduced need for postoperative adjustments. It is possible that surgeons with less experience performing and managing trabeculectomy would experience outcomes that are not as favourable as those obtained in this study and closer to those achieved with the MicroShunt. The relative performance of MicroShunt might be better in surgeons with limited trabeculectomy experience, but this needs to be confirmed with future studies.
Study Limitations
This study had several limitations. Although subjects were masked for treatment assignment, the masking of surgeons was not feasible. Baseline IOP was < 18 mmHg in 31% of eyes (no washout before surgery), which may have led to relatively high rates of failure in both groups. Therefore, the chosen primary outcome measure may not have been an ideal measure for determining surgical success. This is supported by the finding that the percentage of patients in each group who were medication-free was higher than the percentage who achieved overall surgical success. An additional study limitation may have been the use of MMC-soaked sponges at a fixed concentration for a fixed period. Future studies comparing the MicroShunt and trabeculectomy that use varying concentrations of MMC and different delivery methods (e.g., via injection) may yield different results.
Conclusions
This prospective, randomized, single-masked trial showed that both trabeculectomy and MicroShunt implantation resulted in significant and sustained IOP reduction at year 2, with trabeculectomy continuing to result in greater surgical success based on the primary endpoint.
Acknowledgements
The authors thank Dr. Paul Sidoti of the Icahn School of Medicine at Mount Sinai, for his expert review and contributions to the manuscript. Editorial support was provided by BelMed Professional Resources, with funding from Santen.
References
1. J.E. Cairns Trabeculectomy. Preliminary report of a new method Am J Ophthalmol, 66 (1968), pp. 673-679
2. C. Migdal, R. Hitchings Control of chronic simple glaucoma with primary medical, surgical and laser treatment Trans Ophthalmol Soc U K, 105 (Pt 6) (1986), pp. 653-656
3. P.R. Lichter, D.C. Musch, B.W. Gillespie, et al., CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery Ophthalmology, 108 (2001), pp. 1943-1953
4. S.J. Gedde, W.J. Feuer, K.S. Lim, et al. Primary Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 3 Years of Follow-up Ophthalmology, 127 (2020), pp. 333-345
5. S.J. Gedde, J.C. Schiffman, W.J. Feuer, et al. Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up Am J Ophthalmol, 153 (2012), pp. 789-803
6. S.J. Gedde, L.W. Herndon, J.D. Brandt, et al. Tube versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up Am J Ophthalmol, 153 (2012), pp. 804-814
7. H.B. Dick, T. Schultz, R.D. Gerste Miniaturization in glaucoma monitoring and treatment: a review of new technologies that require a minimal surgical approach Ophthalmol Ther, 8 (2019), pp. 19-30
8. M. Shah Micro-invasive glaucoma surgery – an interventional glaucoma revolution Eye Vis (Lond), 6 (2019), p. 29
9. C. Lavia, L. Dallorto, M. Maule, et al. Minimally-invasive glaucoma surgeries (MIGS) for open-angle glaucoma: a systematic review and meta-analysis PLoS One, 12 (2017), Article e0183142
10. L.E. Pillunat, C. Erb, A.G. Junemann, F. Kimmich Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents Clin Ophthalmol, 11 (2017), pp. 1583-1600
11. G.M. Richter, A.L. Coleman Minimally invasive glaucoma surgery: current status and future prospects Clin Ophthalmol, 10 (2016), pp. 189-206
12. L. Pinchuk, I. Riss, J.F. Batlle, et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma J Biomed Mater Res B Appl Biomater, 105 (2017), pp. 211-221
13. N.D. Baker, H.S. Barnebey, M.R. Moster, et al., INN005 Study Group Ab-externo MicroShunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study Ophthalmology, 128 (2021), pp. 1710-1721
14. World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects JAMA, 310 (2013), pp. 2191-2194
15. T. Shaarawy, M. Sherwood, F. Grehn (Eds.), World Glaucoma Association Guidelines on Design and Reporting of Glaucoma Surgical Trials, Kugler Publications, Amsterdam (2009)
16. S.J. Gedde, J.C. Schiffman, W.J. Feuer, et al. Tube Versus Trabeculectomy Study Group. The Tube Versus Trabeculectomy Study: design and Baseline Characteristics of Study Patients Am J Ophthalmol, 140 (2005), pp. 275-287
17. A. Tanner, F. Haddad, J. Fajardo-Sanchez, et al. One-year surgical outcomes of the PreserFlo MicroShunt in glaucoma: a multicentre analysis Br J Ophthalmol, 107 (2023), pp. 1104-1111
18. R. Bhayani, J.M. Martinez de la Casa, M. Figus, et al. Short-term safety and efficacy of Preserflo™ Microshunt in glaucoma patients: a multicentre retrospective cohort study Eye (Lond), 37 (2023), pp. 644-649
19. A.M. Fea, G.L. Laffi, E. Martini, et al. Effectiveness of MicroShunt in patients with primary open-angle and pseudoexfoliative glaucoma: a retrospective European multicenter study Ophthalmol Glaucoma, 5 (2022), pp. 210-218
20. H.J.M. Beckers, F. Aptel, C.A.B. Webers, et al. Safety and effectiveness of the PRESERFLO® MicroShunt in primary open-angle glaucoma: results from a 2-year multicenter study Ophthalmol Glaucoma, 5 (2022), pp. 195-209
21. L.M.J. Scheres, S. Kujovic-Aleksov, W.D. Ramdas, et al. XEN® Gel Stent Compared to PRESERFLO™ MicroShunt Implantation for primary open-angle glaucoma: Two-year Results Acta Ophthalmol, 99 (2021), pp. e433-440
22. S.J. Gedde, J.C. Schiffman, W.J. Feuer, et al. Tube Versus Trabeculectomy Study Group. Three-year follow-up of the Tube Versus Trabeculectomy study Am J Ophthalmol, 148 (2009), pp. 670-684
23. S.J. Gedde, J.C. Schiffman, W.J. Feuer, et al. Treatment Outcomes in the Tube Versus Trabeculectomy Study after One Year of Follow-up Am J Ophthalmol, 143 (2007), pp. 9-22
24. S.J. Gedde, W.J. Feuer, W. Shi, et al. Primary Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 1 Year of follow-up Ophthalmology, 125 (2018), pp. 650-663
25. M.B. Schlenker, G.M. Durr, E. Michaelov, I.I.K. Ahmed Intermediate outcomes of a novel standalone ab externo SIBS Microshunt with mitomycin C Am J Ophthalmol, 215 (2020), pp. 141-153
26. F. Ederer, D.A. Gaasterland, L.G. Dally, et al. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results Ophthalmology, 111 (2004), pp. 651-664
27. E. Bakhtiari Diminished returns in Europe: Socioeconomic status and ethno-racial health disparities across 30 countries in the European Social Survey J Racial Ethn Health Disparities, 9 (2022), pp. 2412-2426
28. M.V. Boland, K.J. Corcoran, A.Y. Lee Changes in the performance of glaucoma surgeries 1994 through 2017 based on claims and payment data for United States Medicare beneficiaries Ophthalmol Glaucoma, 4 (2021), pp. 463-471
Credits:
Panarelli JF, Moster MR, Garcia-Feijoo J, Flowers BE, Baker ND, Barnebey HS, Grover DS, Khatana AK, Lee B, Nguyen T, Stiles MC, Sadruddin O, Khaw PT; INN005 Study Group. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: Two-Year Results from a Randomized, Multicenter Study. Ophthalmology. 2024 Mar;131(3):266-276. doi 10.1016/j.ophtha.2023.09.023. Epub 2023 Sep 27. PMID: 37769852.