Laura-Jane E Smith, consultant respiratory physician1, Ruhi Bhugra, advanced pharmacist in respiratory and acute medicine2,
Reem Y Kelani, musician, broadcaster, translator, and patient with asthma, James Smith, assistant director of public health studies3
1 King’s College Hospital, London, UK
2Lister Hospital, East and North Hertfordshire NHS Trust, Stevenage, UK
3Public Health Education Group, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
Correspondence to: L-J Smith laura-jane.smith@nhs.net
What you need to know
- Hydrofluorocarbon propellants used in pressurized metered dose inhalers (pMDIs) disproportionately contribute to healthcare’s environmental impact
- Reduced use of pMDIs improves planetary outcomes as well as clinical outcomes for patients
- Whenever clinically appropriate, consider low-carbon inhalers (dry powder or soft mist) rather than high-carbon pMDIs
- Seek opportunities to review asthma care at every consultation
Asthma affects over 260 million people and causes more than 460 000 premature deaths annually worldwide.1 There is variation in asthma care and its carbon footprint globally: the UK, for example, has a high hospital admission rate and mortality for asthma compared with other high-income countries,1,2 and it has a high carbon footprint from inhalers. At the centre of these two problems is an over-reliance on pressurized metered dose inhalers (pMDIs)—short-acting β agonist (SABA) reliever pMDIs in particular3 but also inhaled corticosteroid preventer pMDIs.4,5 There is no clinical rationale for this inter-country variation, and we, therefore, use the UK as an example of a country where there are many opportunities for change. The principles that we present apply globally.
What is the problem?
Widespread use of inhalers with high carbon footprints
The National Health Service (NHS) in England is the only healthcare system in which emissions have been comprehensively calculated, and in this system, 13% of the emissions under its direct control (excluding supply chain emissions) are due to inhalers used to treat asthma and other airway diseases.6 This equates to 3% of the total NHS carbon footprint.
SABA inhalers contribute 67% of England’s inhaler carbon footprint,7 and 70% of all inhalers issued in England are pMDIs, (a higher proportion than in many other countries in Europe, such as only 13% of inhalers used in Sweden being pMDIs).8 Within England, variation with the proportion of corticosteroid preventer inhalers that are pMDIs has also been demonstrated; this ranges from 37% in North Tyneside to 70% in North East Lincolnshire.9
High SABA use is also associated with poor clinical outcomes, as shown in a confidential inquiry investigating deaths from asthma or anaphylaxis in the four UK nations between February 2012 and January 2013.2 This confirms that there are two strong incentives for changing asthma care practice: to reduce morbidity and mortality and to reduce the impact of asthma care on the environment.
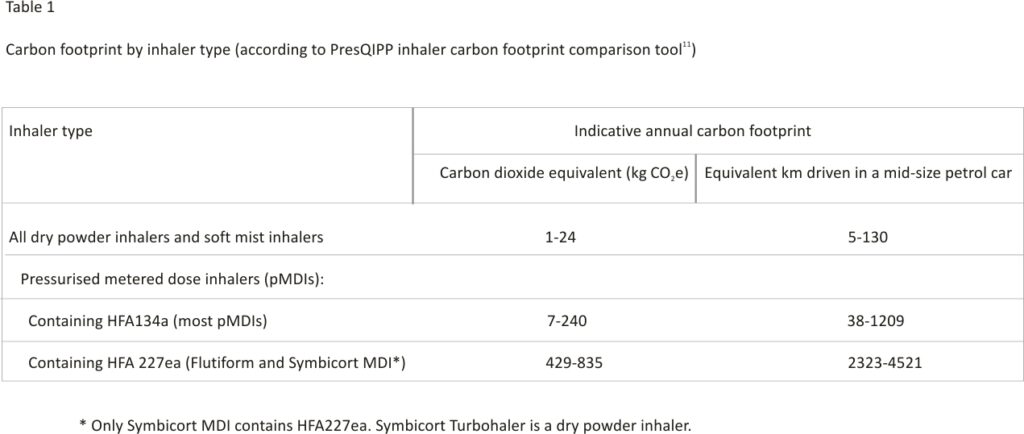
Following a move away from chlorofluorocarbon (CFC) propellants, as part of the global commitment laid out in the 1989 Montreal Protocol on Substances that Deplete the Ozone Layer,10 pMDIs now contain hydrofluorocarbons (HFCs). These are over 1000 times more potent as greenhouse gases than carbon dioxide (CO2).8,11 Although the 2019 Kigali Amendment added HFCs to the Montreal Protocol,12 exemptions are in place for medical uses such as inhalers. Dry powder inhalers (DPIs) and soft mist inhalers (SMIs) have substantially lower carbon footprints than pMDIs as they do not contain HFCs. 8,11 Although there is no standardized method for measuring the carbon footprint of inhalers, best estimates support a broad categorization based on HFC propellant gas into low (inhalers that do not use HFC gases), moderate (inhalers that use the HFC named HFA134a), and high carbon inhalers (inhalers that use the HFC named HFA227ea) (see table 1).11
Overdiagnosis and underdiagnosis of asthma
Asthma is both underdiagnosed and overdiagnosed—patients are commonly given a SABA inhaler for breathlessness or wheezing without diagnostic pulmonary function tests (peak flow diary, spirometry with reversibility, bronchial provocation test, or exhaled nitric oxide).13 A study of NHS prescription data from England in 2017 found that up to 30% of patients in England with a diagnostic label of asthma did not have the disease.14 These and other patients may be using SABA inhalers without clinical benefit, with a high environmental cost.
Underdiagnosis also exists and increases the risks of daily symptoms, exacerbations, and airway remodeling. Questionnaires and objective testing of 10 000 randomly selected people aged 14-44 years in Copenhagen identified 493 with “definite asthma”; of these, 50% had not been diagnosed previously.15 Survey data from 192 young adults entering US military service who had respiratory symptoms on exercise, suggested that, of those diagnosed with asthma at enrolment (using spirometry in all and bronchial provocation testing in 67%), a diagnosis of asthma had never been previously considered in 30%.16
Lack of regulation and incentives that support appropriate inhaler use, reuse, and disposal
Many inhalers do not have dose counters, which means there is a risk of patients throwing away partly used inhalers prematurely.17 Most inhalers are disposed of in domestic waste and, because of the lack of large-scale recycling schemes globally, end up in landfill where their HFCs are leaked into the atmosphere. Additionally, very few inhalers are reusable or refillable. The Respimat (tiotropium bromide) SMI has a reusable dispenser, and refills are available18; however, the entire inhaler is often re-prescribed rather than just the refill cartridge on its own.
Factors such as plastic and metal pollution also contribute to environmental damage, but carbon footprint is the best-described environmental impact of inhalers.19
What are the solutions?
Look for opportunities to optimize clinical care
Ensure diagnoses are correct (see box 1). Global Initiative for Asthma (GINA) and National Institute for Health and Care Excellence (NICE) guidelines recommend offering spirometry with reversibility, peak flow diary monitoring, and fractional exhaled nitric oxide (FeNO), where available, to both newly diagnosed patients and existing patients who are taking SABA alone. Bronchial provocation testing is recommended when there remains diagnostic doubt. However, we acknowledge that, in areas with limited healthcare infrastructure and resources, making an accurate diagnosis can be more challenging if the availability of spirometry and peak flow testing is limited.20
While we advocate for objective pulmonary function testing, we advise that, when clinicians are confident about a diagnosis of asthma, patients can be treated with inhaled corticosteroid or corticosteroid plus long-acting β agonist (LABA) while waiting for objective pulmonary function testing; and that a peak flow diary and/or eosinophilia on full blood count can be considered as adequate confirmation if objective pulmonary function testing is not available.
- If asthma is confirmed, use shared decision-making to create a self-management plan, and transition to a corticosteroid or corticosteroid-LABA inhaler as the primary medication, as per guidelines from GINA.21
- Identify patients who are issued more than three SABA inhalers a year and
- Optimize their treatment in line with GINA and British Thoracic Society guidelines, ensuring they are receiving the right drug, device, and dose to control disease
- Optimize inhaler technique—in person ideally or via video, supported by specialist nurses or pharmacists— and increase the use of maintenance and reliever treatment (MART) and anti-inflammatory reliever (AIR) regimens with the aim of eliminating SABA use in appropriate patients (see box 2).22,23 Additional training may be required so all professionals can reliably assess and teach inhaler technique.24,25
Box 1
The hypothetical case where asthma diagnosis is incorrect
- Clinical scenario—An adult patient has clinical features that are not suggestive of asthma and has perceived no benefit from pMDI medications. Pulmonary function testing revealed no reversibility and low FeNO. Breathing pattern disorder was diagnosed, and pMDI medications were weaned over time, then stopped completely. FeNO was rechecked and remained low.
- Previous medication regimen— Clenil modulate (beclomethasone dipropionate) pMDI 200 μg 2 puffs twice daily, and Salamol (salbutamol) pMDI 100 μg 1-2 puffs as required (patient was using this twice daily).
- New medication regimen— Alternative management strategies for breathing pattern disorder employed.
- Estimated carbon savings per year— 83 kg CO2e (equivalent to ~418 km driven (London to Newcastle upon Tyne (UK) or Central Park New York to The White House, Washington (USA) in a petrol car.
pMDI = pressurised metered dose inhaler, FeNO = fractional exhaled nitric oxide, CO2e = carbon dioxide equivalent.
Box 2
The hypothetical case where MART therapy has clinical and environmental benefits
- Clinical scenario—On the annual review, an adult patient is found to be over-relying on SABA and to be able to use the technique appropriate for DPI (that is, able to take a quick deep breath). The patient was offered MART therapy.
- Previous medication regimen — Symbicort (budesonide/formoterol) pMDI 100/6 1 puff twice daily and Ventolin (salbutamol) pMDI 1 puff as required.
- New medication regimen— Symbicort Turbohaler (budesonide/ formoterol) 100/6 1 puff twice daily maintenance and 1 puff as required.
- Estimated carbon savings per year —220 kg CO2e (equivalent to ~1100 km (Aberdeen to Penzance) driven in a petrol car).
MART = maintenance and reliever treatment, SABA = short acting β agonist, DPI = dry powder inhaler, pMD I = pressurized metered dose inhaler, CO2e = carbon dioxide equivalent.
These measures will reduce the carbon footprint of asthma care even before any changes to inhaler devices are considered.
- Other opportunities to optimize treatment, as outlined above, include when patients present with exacerbations, repeat prescription requests, and during routine asthma reviews.
- Within shared decision-making conversations, discuss non-pharmacological factors such as allergen and air pollution exposure, physical activity, immunization, and psychosocial factors.
- If patients have exacerbations despite optimized treatment, consider assessment for biologic therapies such as omalizumab, mepolizumab, and reslizumab where available.26
Consider prescribing lower-carbon inhalers
- Encourage practitioner behavior change to favor DPIs (and SMIs) over pMDIs.19, 27 A post hoc analysis of 2236 patients in England showed reduced asthma symptoms and greater productivity in daily activities in patients switching from pMDI to DPI.27
- Assess for suitability to use DPIs. DPIs require a deep, quick inhalation and may not be suitable for all patients. Young children and frail older adults, in particular, may not have the technique or inspiratory capacity to achieve this (consider objective testing with a peak flow measuring device if there is doubt). In one UK asthma service, 93.7% of adult patients achieved the necessary peak inspiratory flow for high-resistance DPIs.28 A review of studies across Europe, Japan, Argentina, and the US that included children as young as 3 years old concluded that the evidence supports the efficacy of DPI in treating asthma and COPD irrespective of patient age, even during acute exacerbations.29 There is international variation in the age deemed suitable for DPI use: for example, in the UK this is age 12 years, whereas in Finland children as young as 6 years are recommended DPIs.30
- When choosing inhalers, make shared decisions with patients after discussing the patient’s ability to use the device effectively, preference, their ability to maintain effective inhaler technique, and the environmental impact. This is reflected in a NICE decision aid on asthma inhalers and climate change.31 Avoid blanket switches (where patients are not involved in the decision, merely informed of the switch), which include a change of device or active ingredient, as these can be unsafe and disempowering for patients.
- In a survey of 12 145 UK asthma patients, although 65% were unaware of the carbon footprint of pMDIs, 60% of pMDI users would consider changing devices for environmental reasons and 85% thought asthma patients should be encouraged to use more environmentally friendly inhalers.32 However, always discuss environmental impact collaboratively and sensitively. It is important that patients do not stop their treatment due to concerns about its environmental impact. It is the responsibility of healthcare systems, not individuals, to reduce the environmental impact of care. Be warned that concern about clinical risks from changes in practice that are not evidence-based can lead to inaction and persisting poor clinical and environmental outcomes.13, 3
- Consider cost as part of a wider shared decision-making process, as it might be a barrier to change, although many inhaler switches are at least cost-neutral. If a switch to a DPI is more expensive, consider the full cost of care14 because, if control is improved with increased use of inhaled corticosteroid, then any increased cost may be offset by the reduced need for multiple SABAs and by reduced exacerbations. Nevertheless, in some countries, including where patients directly purchase their medications, the cost of DPIs can be a barrier.34
Consider opportunities to reduce the carbon footprint of asthma care even for those remaining on pMDIs
- Prescribe fewer puffs of a higher-strength inhaler as this reduces the amount of propellant used (see box 3).
- Prescribe a SABA pMDI with a lower carbon footprint: one which uses a low volume of propellant (such as Salamol or Airomir) in place of inhalers that use a high volume of propellant (such as Ventolin).
- Avoid the highest carbon footprint inhalers (those containing propellant HFA227ae, see Table 1) unless no alternative exists.
- Prioritise non-pharmacological interventions such as allergen and air pollution avoidance, physical activity, and immunization.
- Reduce the frequency of automatic repeat prescriptions for SABA inhalers, or change prescriptions to be available only on request.
- Encourage patients to use every dose in their inhalers (facilitated by dose counters on inhalers).
Box 3
The hypothetical case where environmental impact is reduced for a patient who requires pMDI
- Clinical scenario—An adult patient with no recent exacerbations and who is open to change in order to reduce the environmental impact of their asthma care is found to be able to use pMDI most effectively.
- Previous medication regimen— Qvar (beclomethasone diproprionate) pMDI 50 μg 2 puffs twice a day and Ventolin (salbutamol) pMDI 100 μg 1 puff as required.
- New medication regime—Qvar (beclomethasone diproprionate) pMDI 100 μg 1 puff twice daily and Airomir (salbutamol) pMDI 100 μg 1 puff as required.
- Estimated carbon savings per year—404 kg CO2e (equivalent to ~2035 km (Oxford, UK, to Rome, Italy) driven in a petrol car).
pMDI = pressurised metered dose inhaler, CO2e = carbon dioxide equivalent.
Campaign for system changes, innovation of inhaler design, and implementation of recycling schemes
- Engage national and regional leaders to drive change as international policy alone is unlikely to deliver the urgent change required.10,12,35
- Implement schemes for recycling or incineration of all inhalers to ensure any remaining HFC is salvaged or destroyed. In England, community pharmacies are required to advise patients to return inhalers to pharmacies for disposal (currently, incineration is not yet linked to recycling).36 A pilot postal scheme in Leicestershire, England had 20 049 inhalers returned and recycled over 12 months, saving an estimated 119.3 tonnes of CO2e.37
- Actively amplify the voice of clinicians and patients—pharmaceutical companies have an opportunity to innovate device designs to reduce environmental impact but are unlikely to do so without this demand. Pharma-led solutions could include the use of lower GHG HFC propellants in pMDIs, such as HFA 152a38 and HFO-1234ze39 which are in development; reducing plastic use (for all inhalers) to protect ocean health; and the adoption of circular economy principles in which waste and pollution are designed out of inhaler devices and materials can be recycled and reused.40
Place a greater focus on asthma prevention
- Collaborate with patients to advocate for policies and interventions to prevent asthma by reducing indoor and outdoor air pollution, strengthening tobacco control policies, improving housing, and addressing all causes of health inequalities.
- Work to match resource allocation, particularly in terms of quality improvement project support, staff time, and research funding, with the urgency of the need for change.
Global efforts to reach net zero for asthma care
- To reach net zero, low-cost, low-carbon inhalers will need to be made available in all countries. The WHO includes budesonide and budesonide-formoterol in the list of essential medicines for asthma. There are ongoing efforts to make such medications available in functioning health systems at all times, in appropriate dosage forms, of assured quality, and at prices individuals and health systems can afford.41
- A retrospective analysis of data from hospitalizations in the Brazilian public health system of individuals with asthma aged from 1 to 49 years, showed that, since the provision of free access to beclomethasone and salbutamol inhalers in 2011, there has been a reduction in hospitalizations for asthma.42
- Inhaled corticosteroid is under-prescribed and underused in India, contributing to an estimated 42% of global asthma deaths.43 An editorial in Lung India suggests that, if corticosteroid or corticosteroid-formoterol inhalers were to come under the Drug Price Control Order Act in India, access to them would improve.44,45
- Both NHS England6 and Scotland46 have committed to net zero by 2040 for the emissions they control. Unfortunately, NHS England recently removed financial incentives that rewarded general practitioners for prescribing lower-carbon inhalers.47
- National and local prescribing guidelines are an opportunity to address climate change in clinical practice. National guidance in Finland,30 New Zealand,48, and the UK specifically recommend taking into account the carbon footprint of inhalers when prescribing for asthma. In Finland, dry powder inhalers are recommended as the primary form of administration for most school-age children and adults.
Information resources for clinicians and patients
- Greener Practice. High-quality and low-carbon asthma care (https:// www.greenerpractice.co.uk/high-quality-and-low-carbon-asthma-care/)
- ERS. Environment and health (https://www.ersnet.org/advocacy/environment-and-health/)
- PrescQIPP. Bulletin 295: Inhaler carbon footprint (https://www. prescqipp.info/our-resources/ bulletins/bulletin-295-inhaler-carbon-footprint/)
- Centre for Sustainable Healthcare. Sustainable respiratory care network (https://networks.sustainable healthcare.org.uk/network/sustainable-respiratory-care)
Patients
- Asthma and Lung UK (https:// www.asthma.org.uk/)
- NICE. NG80 Asthma inhalers and the environment patient decision aid: Asthma inhalers and climate change (https://www.nice.org. uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573)
- Green Inhaler (https://greeninhaler. org/)
- European Lung Foundation. Healthy lungs for life (https://europeanlung. org/en/projects-and-campaigns /healthy-lungs-for-life/)
Education into practice
- What dosing regimens and lower carbon footprint options could you offer to asthma patients who continue to need pressurised metered dose inhalers?
- How do you create asthma care plans that optimally manage symptoms with a reduced need for short-acting β agonist inhalers?
- What could your practice do to campaign for changes to prevent asthma?
How patients were involved in the creation of this article
A patient with asthma is a co-author on this article. They agreed the outline of the article and suggested a greater emphasis on practical steps, and the importance of shared decision making. They reviewed the initial draft and made multiple changes to structure and content, in particular suggesting emphasising the value of specialist nurses and pharmacists, as well as changes to language around patient empowerment. They reviewed all suggested resources to ensure they were appropriate and valuable from a patient perspective.
In addition, as a result of an external patient review, arranged by the BMJ we made it clearer that there are multiple ways to reduce environmental impact, including options for different global settings. We also explicitly stated that patients should not stop their inhalers and emphasised that poorly controlled asthma carries the greatest environment burden. We highlighted more strongly that it is the responsibility of the system not the individual to reduce the environmental impact of care.
How this article was created
To write this clinical update we started with the personal archives of references on asthma care and environmental impact of James Smith and LJ Smith. We supplemented this with a Medline search using terms (environmental OR carbon OR net zero) AND inhaler. NICE, BTS, SIGN, and GINA guidelines were searched for reference to environmental impact. The Cochrane Database was searched for reference to inhalers. International guidelines were sought by searching respiratory society websites.
Footnotes
Contributors: JS had the initial idea for the article and its core themes. All authors met to discuss and agree key messages and article sections and provided initial references. JS wrote a first draft on the basis of these discussions and all authors commented, substantially altering the text before initial submission. LJS conducted a Medline search. LJS led on responding to reviewer’s comments. All authors commented on subsequent reviews. RB provided calculations of carbon footprint that were independantly calculated by JS and LJS. RK made material changes to language and emphasis, foregrounding the patient voice. All authors reviewed the final manuscript.
Competing interests:
The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: JS and LJS are members of NHS England Inhalers Group. Further details of The BMJ policy on financial interests is here: https://www.bmj.com/sites/default/ files/attachments/resources/2016/ 03/16-current-bmj-education-coi-form. pdf.
Patient consent:
Not required (cases are hypothetical).
References
1. Vos T, Lim SS, Abbafati C, et al., GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet2020;396: 1204-22. doi:10. 1016/S0140-6736(20)30 925-9pmid:3306 9326
2. Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD). 2015. https://www.rcplondon. ac.uk/projects/outputs/why-asthma-still-kills.
3. The Global Asthma Report. 2018. http://globalasthmare port.org/.
4. Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: A green challenge and a golden opportunity. Br J Clin Pharmacol2022;88:3016-22. doi: 10.1111/bcp.15135pmid:3471 9810
5. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future HealthcJ2018;5:88-93. doi:10.7861/futurehosp.5-2-88 pmid:31098540
6. NHS England. Delivering a ‘Net Zero’ National Health Service. 2022. https://www.england.nhs.uk/greenernhs/publication/delivering-a-net-zero-national-health-service/.
7. Wilkinson AJK, Menzies-Gow A, Sawyer M, et al. S26 An assessment of short-acting β2-agonist (SABA) use and subsequent greenhouse gas (GHG) emissions in five European countries and the consequence of their potential overuse for asthma in the UK. Thorax2021;76(Suppl 1):A19.
8. Janson C, Henderson R, Löfdahl M, Hedberg M, Sharma R, Wilkinson AJK. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax2020;75:82-4. doi:10.1136/thoraxjnl-2019-213 744 pmid:31699805
9. OpenPrescribing. Explore England’s prescribing data. 2022. https:// openprescribing.net/.
10. UN Environment Programme. The Montreal Protocol on Substances that Deplete the Ozone Layer. 2020. https://ozone.unep.org/treaties/ montreal-protocol.
11. PresQIPP. Bulletin 295: Inhaler carbon footprint. 2022. https:// www.prescqipp.info/our-resources /bulletins/bulletin-295-inhaler-carbon-footprint/.
12. UN Environment Programme. The Montreal Protocol on Substances that Deplete the Ozone Layer. Decision XXVIII/1: Further Amendment of the Montreal Protocol. https://ozone.unep. org/sites/default/files/2019-04/Original_depositary_notification_english_version_with_corrections.pdf.
13. Gupta S, Thériault G. Do not diagnose or routinely treat asthma or chronic obstructive pulmonary disease without pulmonary function testing. BMJ2023;380: e072834. doi:10.1136/bmj-2022-072834 pmid:36940980
14. Wilkinson AJK, Braggins R, Steinbach I, Smith J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019;9:e 028763. doi:10.1136/bmj open-2018-028763 pmid:3166 2306
15. Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med2006; 100:354-62. doi:10.1016/j.rmed. 2005.05.012 pmid:16005621
16.Nish WA, Schwietz LA. Underdiagnosis of asthma in young adults presenting for USAF basic training. Ann Allergy 1992;69: 239-42.pmid:1524281
17. Fullwood I, Evans T, Davies B, et al. Do you know when the inhaler is empty?Arch Dis Child2022;107:9 02-5. doi:10.1136/archdischild-2022-324027 pmid:35551051
18. National Institute for Health and Care Excellence. British National Formulary (BNF). Tiotropium: Patient and carer advice. https:// bnf.nice.org.uk/drugs/tiotropium/.
19. Fulford B, Mezzi K, Aumônier S, Finkbeiner M. Carbon footprints and life cycle assessments of inhalers: a review of published evidence. Sustainability 2022;14: 7106doi:10.3390/su14127106 .
20. Kibirige D, Kampiire L, Atuhe D, et al. Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective. BMC Pulm Med2017;17:179. doi:10. 1186/s12890-017-0527-y pmid: 29216852
21. Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am J Respir Crit Care Med2022;205:17-35. doi:10.11 64/rccm.202109-2205PP pmid: 34658302
22. Canonica GW, Paggiaro P, Blasi F, et al. Manifesto on the overuse of SABA in the management of asthma: new approaches and new strategies. Ther Adv Respir Dis 2021;15:17534666211042534. doi:10.1177/1753466621104253
23. Nannini LJ, Neumayer NS, Brandan N, Fernández OM, Flores DM. Asthma-related hospitalizations after implementing SABA-free asthma management with a maintenance and anti-inflammatory reliever regimen. Eur Clin Respir J2022;9:2110706. doi:10.1080/ 20018525.2022.2110706 pmid: 35959199
24. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res2018;19:10. doi:10.11 86/s12931-017-0710-y pmid: 29338792
25. Basheti IA, Qunaibi EA, Hamadi SA, Reddel HK. Inhaler technique training and health-care professionals: effective long-term solution for a current problem. Respir Care 2014; 59:1716-25. doi:10.4187/respcare. 02671 pmid:24962222
26. Congleton J. Asthma: a disease of variability. Drug Ther Bull 2021; 59:50. doi:10.1136/dtb.2020. 000069 pmid:33766921
27. Woodcock A, Janson C, Rees J, et al. Effects of switching from a metered dose inhaler to a dry powder inhaler on climate emissions and asthma control: post-hoc analysis. Thorax2022;77:1187-92. doi:10.1136/thoraxjnl-2021-218088 pmid:35131893
28. Haughney J, Lee AJ, McKnight E, Pertsovskaya I, O’Driscoll M, Usmani OS. Peak inspiratory flow measured at different inhaler resistances in patients with asthma. J Allergy Clin Immunol Pract2021;9:890-6. doi:10.1016/j.jaip.2020.09.026 pmid:33011302
29. Vartiainen VA, Lavorini F, Murphy AC, Rabe KF. High inhaler resistance does not limit successful inspiratory maneuvers among patients with asthma or COPD. Expert Opin Drug Deliv2023;20:385-93. doi:10.10 80/17425247.2023.2179984 pmid:36820500
30. Käyä hoito. Asthma. Valid treatment recommendation. 2022. https:// www.kaypahoito.fi/hoi06030.
31. National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management (NICE guideline Ng80). 2021. Asthma inhalers and climate change. https://www. nice.org.uk/guidance/ng80/resources/asthma-inhalers-and-climate-change-patient-decision-aid-pdf-6727144573.
32. D’Ancona G, Cumella A, Renwick C, Walker S. The sustainability agenda and inhaled therapy: what do patients want? Eur Respir J2021; 58:PA3399. doi:10.1183/1399 3003.congress-2021.PA3 399.
33. IPCC. Global warming of 1.5°C. 2018. https://www.ipcc.ch/sr15 /.
34. Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ2007; 85:279-88. doi:10.2471/BLT. 06.033647 pmid:17546309
35. Pritchard JN. The Climate is Changing for Metered-Dose Inhalers and Action is Needed. Drug Des Devel Ther2020;14:3043-55. doi:10. 2147/DDDT.S262141 pmid:328 01643
36. Community Pharmacy England. Disposal of unwanted medicines. 2022. https://cpe.org.uk/ national-pharmacy-services/essential-services/disposal-of-unwanted-medicines/.
37. Murphy A, Howlett D, Gowson A, Lewis H. Understanding the feasibility and environmental effectiveness of a pilot postal inhaler recovery and recycling scheme. NPJ Prim Care Respir Med2023; 33:5. doi:10.1038/s41533-023-00327-w pmid:3668 1666
38. Zephex. Zephex 152a. 2022. https://www.zephex.com/zephex-152a/.
39. AstraZeneca. AstraZeneca progresses the Ambition Zero Carbon programme with the Honeywell partnership to develop next-generation respiratory inhalers. 2022. https://www. astrazeneca.com/media-centre /press-releases/2022/astra zeneca-progresses-ambition-zero-carbon-programme-with-honeywell-partnership-to-develop-next-generation-respiratory-inhalers. html.
40. Colorado HA, Gutiérrez Velásquez EI, Monteiro SN. Sustainability of additive manufacturing: the circular economy of materials and environmental perspectives. J Mater Res Technol2020;9:8221-34 doi:10.1016/j.jmrt.2020.04.062.
41. World Health Organization. WHO model list of essential medicines – 22nd list, 2021. 2021. https://www. who.int/publications-detail-redirect/WHO-MHP-HPS-EML-2021.02.
42. Comaru T, Pitrez PM, Friedrich FO, Silveira VD, Pinto LA. Free asthma medications reduce hospital admissions in Brazil (Free asthma drugs reduce hospitalizations in Brazil). Respir Med2016;121:21-5 doi:10.1016/j.rmed.2016.10.008 pmid:27888987
43. Salvi S, Madas S, Ghorpade D, Gadhave S, Barne M. Is underuse of Inhaled Corticosteroids for Asthma in India contributing to 42% of global asthma deaths? Lung India2022;39:331-6. doi:10.4103/ lungindia.lungindia_600_21 pmid:35848664
44. Sovani MP, Martin MJ. Inhaled Corticosteroids for asthma treatment in India: An urgent and radical rethink needed. Lung India 2022;39: 311-2. doi:10.4103/lungindia. lungindia_311_22 pmid:358486 59
45. Sovani MP, Martin MJ. Inhaled Corticosteroids for asthma treatment in India: An urgent and radical rethink needed. Lung India2022; 39:311-2. doi:10.4103/lungindia. lungindia_311_22 pmid:35848 659
46. Scottish Government. NHS Scotland climate emergency and sustainability strategy: 2022-2026 2022. http:// www.gov.scot/publications/nhs-scotland-climate-emergency-sustainability-strategy-2022-2026.
47. NHS England. Changes to the GP Contract 2023. 2023. https:// www.england.nhs.uk/long-read/ changes-to-the-gp-contract-in-2023-24/.
48. Beasley R, Beckert L, Fingleton J, et al. Asthma and Respiratory Foundation NZ Adolescent and Adult Asthma Guidelines 2020: a quick reference guide. https:// www.nzrespiratoryguidelines.co.nz/uploads/8/3/0/1/83014052/arfnz_adolescent_and_adult_asthma_guidelines_.pdf.
CREDITS: Smith L E, Bhugra R, Kelani R Y, Smith J. Towards net zero: asthma care BMJ 2023; 381 :e072328 doi:10. 1136/bmj-2022-072328