Donatien Serge Mbaga, Sebastien Kenmoe, Cyprien Kengne-Ndé, Jean Thierry Ebogo-Belobo, Gadji Mahamat, Joseph Rodrigue Foe-Essomba, Marie Amougou-Atsama, Serges Tchatchouang, Inès Nyebe, Alfloditte Flore Feudjio, Ginette Irma Kame-Ngasse, Jeannette Nina Magoudjou-Pekam, Lorraine K. M. Fokou, Sara Honorine Riwom Essama
Abstract
Introduction
Africa denotes unique facies for hepatocellular carcinoma (HCC) characterized by a conjunction of low sensitization, restricted access to diagnosis and treatment, and associated with the highest incidence and mortality in the world. We investigated whether hepatitis B (HBV), C (HCV) and D (VHD) viruses were etiological agents of HCC in Africa.
Methods
Relevant articles were searched in PubMed, Web of Science, African Index Medicus, and African Journal Online databases, as well as manual searches in relevant reviews and included articles. Analytical studies from Africa evaluating the association between HCC development and HBV, HCV, and HDV were included. Relevant studies were selected, data extracted, and the risk of bias assessed independently by at least 2 investigators. The association was estimated using odds ratios (OR) and their 95% confidence interval (95% CI) determined by a random-effects model. Sources of heterogeneity were determined by subgroup analyses.
Results
A total of 36 case-control studies were included. With controls having non-hepatic disease, the overall results suggested a significantly increased risk of HCC in patients with HBV (HBeAg (OR = 19.9; 95% CI = [3.7–105.2]), HBsAg (OR = 9.9; 95%) CI = [6.2–15.6]) and DNA (OR = 8.9; 95% CI = [5.9–13.4]); HCV (Anti-HCV (OR = 9.4; 95% CI = [6.3–14.0]) and RNA (OR = 16.5; 95% CI = [7.8–34.6]); HDV (Anti-VHD, (OR = 25.8; 95% CI = [5.9–112.2]); and HBV/HCV coinfections (HBV DNA/HCV RNA (OR = 22.5; 95% CI = [1.3–387.8]). With apparently healthy controls, the overall results suggested a significantly increased risk of HCC in patients with HBV (HBsAg, (OR = 8.9; 95% CI = [6.0–13.0]); HCV (Anti-HCV, (OR = 7.7; 95% CI = [5.6– 10.6]); and HBV/HCV coinfections (HBsAg/Anti-HCV (OR = 7.8; 95% CI = [4.4–13.6]) Substantial heterogeneity and the absence of publication bias were recorded for these results.
Conclusions
In Africa, HBV/HCV coinfections and HBV, HCV, and HDV infections are associated with an increased risk of developing HCC. The implementation of large-scale longitudinal and prospective studies including healthy participants to search for early biomarkers of the risk of progression to HCC is urgently needed.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and the third leading cause of death due to malignancy worldwide 1. HCC incidence is continually increasing with more than 900 thousand new cases and as many deaths recorded worldwide in 2020 2. HCC rates vary widely across the world with the greatest burden reported in Southeast Asia, East Asia, and sub-Saharan Africa 1. Almost 80% of the morbidity and mortality due to HCC is attributed to developing countries 3.
Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) are the main etiologic agents of HCC 4,5. More than half of the HCC cases are attributable to HBV while about 25% are attributable to HCV. Controversial results have been reported on the increased risk of HBV/HCV coinfections compared to HBV or HCV mono infections 6–9. The role of Hepatitis D Virus (HDV) and occult hepatitis B in the development of HCC has also been demonstrated 10,11. The etiological factors of HCC vary considerably across regions of the world and indicate a strong disparity in the distribution of incidences of HCC 12. The areas with high HBV prevalence are also those with the highest rates of HCC. Africa, which is a region with high endemicity for HBV, thus has the highest incidences of HCC with more than 15 incident cases per 100 thousand inhabitants 13–16. Key reviews, which included very few African studies, reported the importance of genotyping and mutations of interleukin-6 and HBV as biomarkers for early identification of the risk of progression to HCC 17–19. Africa is a unique region plagued by limited awareness, late diagnosis, and limited access to care and treatment for HCC 20. The critical burden of HCC in Africa is mostly related to late diagnosis and limited access to treatment 21–23. Precise synthesis of the roles of the main HCC viral etiological factors (HBV, HCV, and HDV) would be important to enlighten health decision-makers on preventive and early diagnosis methods specific to the African region.
We conducted a systematic review to determine whether HBV, HCV, HDV infections, and HBV/HCV coinfections are associated with an increased risk of developing HCC in Africa.
Methods
Literature search
The electronic literature search was performed for studies published from databases inception through February 2020 and updated in March 2021. Searches were conducted in Pubmed, Web of Science, African Index Medicus, and African Journal Online. The strategy included the keyword combination for exposure (HBV, HCV, and HDV), outcome (HCC), and context (Africa) (S1 Table). Additional potentially relevant studies were searched manually from the reference list of included articles and relevant reviews. The protocol for this review was declared in the international PROSPERO database (CRD42020181381) and complied with the PRISMA guidelines (S2 Table).
Inclusion and exclusion criteria
Regardless of antiviral therapy status, comparative studies (clinical trials, cohort, and case-control) examining the relationship between infection with HBV, HCV, HDV, and HBV/HCV coinfections and the risk of developing HCC were considered relevant for this review. Only studies in French or English conducted in Africa were considered. We considered all types of HCC diagnosed by clinical, histological, biochemical, and radiological approaches. The controls were healthy people, people with non-hepatic diseases, people with liver cirrhosis, and people with hepatic diseases other than liver cirrhosis (other liver diseases). All the detection techniques for HBV (HBsAg, HBeAg, and HBV DNA), HCV (anti-HCV antibodies and HCV RNA), and HDV (anti-HDV antibodies, AgHDV and HDV RNA) infection markers were considered. Studies excluded were those without control groups, with participant selection bias, with no full text and/or abstracts available, conducted outside of Africa, duplicates, case reports, and reviews.
Selection, data extraction, and risk of bias assessment of included studies
Studies were selected based on a title/abstract screening according to the study inclusion criteria. Full texts of selected studies were reviewed to validate eligibility and data extracted from included studies. Data retrieved were the name of the first author, year of publication, study design, sampling approach, the timing of testing for hepatitis virus infection (retrospective /prospective), country, UNSD region, country income level, period of recruitment of study participants, study context (rural/urban and community /hospital). We also collected the inclusion criteria of participants, the definition of HCC, the inclusion criteria for controls, socio-demographic confounding factors, other non-viral confounding factors known to be associated with the risk of HCC, biochemical parameters of liver, type of hepatic virus (HBV, HCV or HDV), the hepatitis virus detection method, the marker searched for the detection of hepatitis virus, the number of participants and controls and the number of exposed and unexposed subjects. Data for the assessment of the individual risk of bias of studies using the Newcastle-Ottawa method (S3 Table). Discussion and consensus were used to resolve issues in the event of disagreements between investigators.
Statistical analysis
The parameters used to estimate the association between viral hepatitis and the risk of developing hepatocellular carcinoma were odds ratios (OR) and the corresponding 95% confidence intervals determined by random effect meta-analysis 24. Quantitative analysis was performed using the packages “meta” and “metaphor” of R software version 4.0.3 software 25,26. Heterogeneity was assessed for each analysis using Cochrane’s Q-test and I2 measurement 27. P-value ≤ 0.10 or I2 ≥ 50% indicates significant heterogeneity. The publication bias was examined by the Egger test and funnel plot, and P-value ≤ 0.10 indicate the presence of a publication bias 28. Sensitivity analysis was performed to evaluate the validity and reliability of overall results on studies with a low risk of bias and those comparable for confounding factors 29.
Results
Study selection
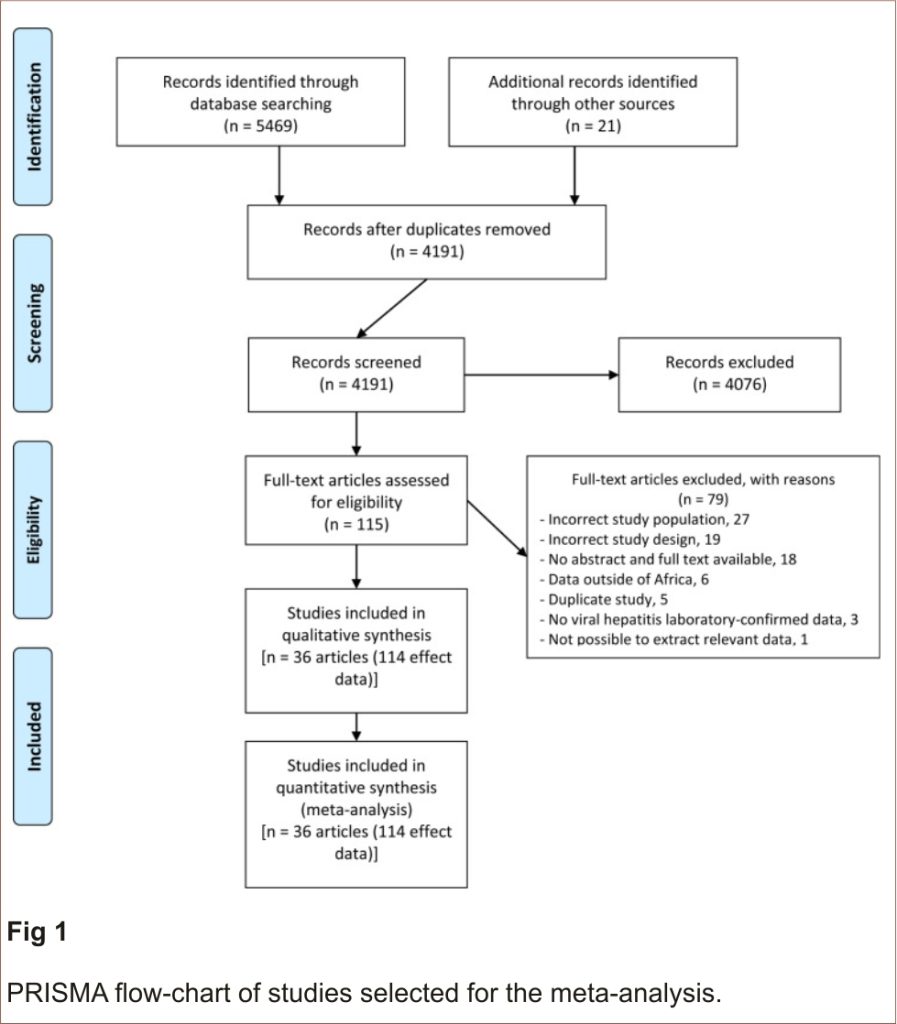
The article selection process is presented in Fig 1. A total of 5469 articles were identified from our electronic literature search and 21 manually from the reviewed bibliography. We excluded 1299 duplicates, 4076 based on title and abstract screening, and 79 for various reasons including wrong study population or design and unavailable article full texts (S4 Table). We thus had a total of 36 studies (114 effect data) that fit the inclusion criteria for this review 30–65.
Included studies characteristics
Bias assessment scores ranged from 3 to 9 and the median was 8 [IQR = 6–8]. Out of the 114-effect data, 75 (65.7%) were of low risk of bias, 37 (32.4%) of moderate risk of bias, and 2 (1.7) of high risk of bias. The risk of bias in the effect data is shown in S5 Table. Participants in the included studies were recruited between 1981 and 2015 and studies published between 1975 and 2020 (S6 Table). All effect data included in this meta-analysis had a case-control design and the majority had non-probability sampling (105/ 114). No study was representative of a national population. The collection of data on hepatitis status was prospective in half of the effect data (56/114). Most of the effect data came from West Africa (52/114) and North Africa (27/114). Most of the included effects data were from The Gambia (19/114), Egypt (18/114), and South Africa (14/114). Half of the effect data came from low-middle income countries (57/114). None of the included study authors stated that the study was conducted in a rural area and most were in a hospital setting (89/114). Controls in effect data were predominantly people with the non-hepatic disease, mostly matched for age and gender. Sixty-two effect data were for HBV, 35 for HCV, 12 for HDV, and 5 for HBV/HCV coinfection. The majority of the effect data used multiple HCC diagnostic approaches including clinical, biochemical, radiological, and histological methods (79/114). More than half of the effect data had confirmed HCC histologically (70/114). The most widely used hepatitis virus detection techniques were radioimmunoassay (26/114), indirect (24/114), direct (19/114) Enzyme-Linked Immunosorbent Assay, and Enzyme immunoassay (16/114). The HBV (HBeAg, HBsAg, and DNA), HCV (Anti-HCV and RNA) and HDV (HDV Ag, Anti HDV, and RNA) markers studies used 4 types of controls including apparently healthy controls, controls with non-hepatic diseases, controls with cirrhosis of the liver and controls with other liver diseases. The most represented markers were HBsAg (48/114), Anti-HCV (33/114), and Ag Delta (8/114) for HBV, HCV, and HDV respectively. The primary characteristics of the endpoint data are presented in the S7 Table.
Meta-analysis Hepatitis B Virus
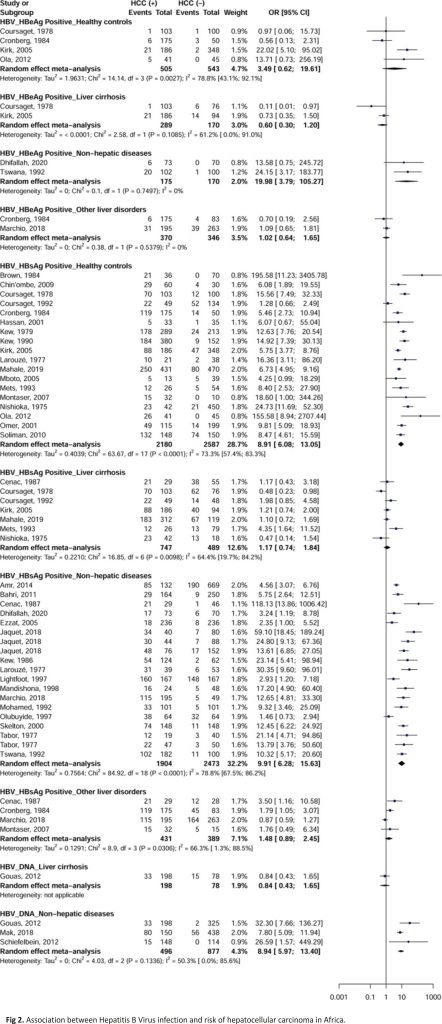
HBeAg (OR = 19.9; 95% CI = [3.7–105.2]), HBsAg (OR = 9.9; 95% CI = [6.2–15.6]) and DNA (OR = 8.9; 95% CI = [5.9–13.4]) for HBV were associated with an increased risk of developing HCC with controls with non-hepatic disease (Fig 2). HBsAg was further associated with an increased risk of developing HCC in apparently healthy controls (OR = 8.9; 95% CI = [6.0–13.0]). No significant difference was observed for HBeAg with apparently healthy controls (OR = 3.4; 95% CI = [0.6–19.6]), controls with liver cirrhosis (OR = 0.6; 95% CI = [0.3–1.2]), and controls with other liver diseases (OR = 1.0; 95% CI = [0.6–1.6]). No significant difference was observed for HBsAg with controls with liver cirrhosis (OR = 1.1; 95% CI = [0.7–1.8]) and controls with other liver diseases (OR = 1.4; 95% CI = [0.8–2.4]). No significant difference was observed for HBV DNA in a study of control with liver cirrhosis (OR = 0.8; 95% CI = [0.4–1.6]). For categories with 3 or more effect data, heterogeneity was significant (I2> 50%) for all analyses according to the types of HBV marker and controls.
Meta-analysis Hepatitis C Virus
Anti-HCV (OR = 9.4; 95% CI = [6.3–14.0]) and RNA (OR = 16.5; 95% CI = [7.8–34.6]) of HCV were associated with increased risk of developing HCC with controls with non-hepatic disease (Fig 3). Anti-HCV was also associated with an increased risk of developing HCC in apparently healthy controls (OR = 7.7; 95% CI = [5.6–10.6]). There were no studies of HCV RNA with apparently healthy individuals, controls with cirrhosis of the liver, and those with other liver diseases. No significant difference was observed for anti-HCV with controls with liver cirrhosis (OR = 1.9; 95% CI = [0.9–3.8]) and controls with other liver diseases (OR = 1.1; 95% CI = [0.7–1.7]). For categories with 3 or more outcome data, except for anti-HCV with apparently healthy controls, heterogeneity was significant (I2> 50%) for the rest of the analyzes by type of marker HCV and controls.
Meta-analysis Hepatitis B Virus/ Hepatitis C Virus co-infections
HBV DNA/HCV RNA (OR = 22.5; 95% CI = [1.3–387.8]) of HBV/HCV coinfection was associated with an increased risk of developing HCC with controls with non-hepatic disease (Fig 4). HBsAg/anti-HCV (OR = 7.8; 95% CI = [4.4–13.6]) of HBV/HCV coinfection were associated with an increased risk of developing HCC with apparently healthy controls. No significant difference was observed for HBsAg/anti-HCV with controls with non-hepatic diseases (OR = 1.8; 95% CI = [0.5–6.6]). Only one category of analyzes on HBV/HCV coinfection had 3 or more effective data and showed no heterogeneity in the estimation of the association between HBsAg/anti-HCV and HCC with apparently healthy controls.
Meta-analysis Hepatitis D Virus
No positive HDV antigen was found in two studies and no meta-analysis was possible. Most of the categories analyzed had less than 3 studies and it is, therefore, difficult to conclude from the results obtained. Anti-HDV was associated with a risk of developing HCC with controls with non-hepatic disease (OR = 25.8; 95% CI = [5.9–112.2], 3 studies) (Fig 5). Only one category of HDV assays had 3 or more effective data and showed no heterogeneity in the estimation of the association between anti-HDV and HCC with controls with the non-hepatic disease.
Publication bias and sensitivity analysis
Egger’s linear regression test found no evidence of significant publication bias for any analysis category with at least 3 effect data (p> 0.005; Table 1). This result was confirmed by funnel plots which showed no asymmetry (S1–S11 Figs). We performed a sensitivity analysis for categories with 3 or more outcome data by selecting only studies with a low risk of bias and studies comparable to confounding factors identified in the included studies. S8 and S9 Tables present the distribution between cases and controls for covariates known to be associated with the risk of HCC. The selection of these studies for sensitivity analysis did not change the overall trends (Table 1). A few exceptions were recorded for certain confounding factors represented by a single study.
Source of heterogeneity examination
In the additional sub-analysis, a study with data from HBeAg infections collected retrospectively (p = 0.001), conducted in The Gambia (p = 0.001), in a low-resource setting (p = 0.001), after the years 2000 (p <0.001) using enzyme immunoassays for HBeAg detections (p = 0.001) had a significantly higher effect of the association between HBeAg and HCC with apparently healthy controls (OR = 22.0; 95% CI = [5.1– 95.0]) (S10 Table) 46. The association between HBsAg and HCC with apparently healthy controls was maintained in all categories of further sub-analysis. This effect, however, varied statistically significantly depending on the country (p = 0.005) and the method of hepatitis detection (p = 0.030). An increase in the effect size between HBsAg and HCC with apparently healthy controls depending on the country’s income level (p = 0.021) was observed. No difference was observed in the subgroup analysis of the effect between HBsAg and HCC with controls with liver cirrhosis. With controls having non-hepatic diseases, the effect between HBsAg and HCC varied significantly by country (p <0.001), UNSD region (effect highest in West Africa, p = 0.001), level of country income (effect higher in low-income countries, p = 0.032) and hepatitis detection technique (effect higher with rapid diagnostic tests, p <0.001). With controls with other hepatic diseases, the effect between HBsAg and HCC varied significantly depending on the timing of data collection on hepatitis virus infection (high effect in prospective studies, p = 0.005), the UNSD region (size of the high effect in West Africa, p = 0.021) and the year of publication (size of the high effect in articles published before 2000, p = 0.021). No difference was observed in the effect analysis subgroups between HBV DNA and HCC with controls with non-hepatic disease. No difference was observed in the subgroup analysis of the effect between anti-HCV and HCC with apparently healthy controls. With controls having liver cirrhosis, the effect between anti-HCV and HCC varied significantly depending on the timing of data collection for hepatitis virus infection (high effect size for retrospective studies, p = 0.014), country (highest effect size in the Gambia, p = 0.049) and the publication year (high effect size in articles published after 2000, p = 0.014). With controls having non-hepatic disease, the effect between anti-HCV and HCC varied significantly by country (p = 0.002) and UNSD region (highest effect size in East Africa, p = 0.006). No significant difference was observed in the analysis subgroups of the effect between anti-HDV and HCC with the controls with non-hepatic disease and HBV/HCV and HCC coinfection with the healthy controls.
Discussion
This review shows a quantitative overview of the relative contribution of HBV, HCV, and HDV in the development of HCC in 20 countries of the 5 African USND regions. As expected, HBV, HCV, and HDV markers were important contributors to the development of HCC with apparently healthy controls or those with non-hepatic disease. These results were confirmed when taking into account multiple confounding factors for the risk of developing HCC such as pesticides, aflatoxin B1, alcohol, smoking, other comorbidities, geographic context, mode of diet, gender, and age. Significant associations were seen in all subgroups of HBsAg, HBV DNA, and anti-HCV markers with apparently healthy controls and those with non-hepatic disease.
The high risk of HCC associated with HBV, HCV, HDV, and HBV/HCV markers observed during this study is consistent with previous systematic reviews conducted at global, regional, and national levels 6–10, 66.
The following information should be taken into account when interpreting the findings in this review. The gold standard for diagnosing HCC is histology. In this review of data from Africa, a context where access to HCC diagnosis is restricted 21–23, only half of the included studies had used histology for the diagnosis of HCC. Difficulty in accessing the diagnosis of HCC in the African context may also have overrepresented patients with liver tumors in the last disease stages in this review. More than half of the included studies used multiple diagnostic approaches for HCC, suggesting a substantial amount of residual heterogeneity that we were not able to address in a subgroup analysis. The majority of included study investigators also did not ascertain the absence of HCC in controls, which suggests the possibility of misclassifying cases as controls. Although we planned to include prospective longitudinal studies in this review, all included studies were retrospective case-control studies. This suggests a potential recall bias, which is further exacerbated by the poor prognosis of cases recruited in the terminal phase of HCC. All the studies that we included had a case-control design with a prospective diagnosis of markers of viral hepatitis B, C, and D for the majority. It is usually accepted that chronic hepatitis is those that have lasted in patients for at least 6 months. Without the participant follow-up data reported in the included studies, we are unable to distinguish acute from chronic hepatitis in the findings of the present review. Due to insufficient or absence of data 40,52, for HBV, HCV, and HDV, we did not assess the role played by occult infections, viral load, mutations, genotypes, and antiviral therapy on the risk of development of the HCC. Our findings are, however, very robust due to 1) a strengthening of the level of confidence for the overall results by a sensitivity analysis including only comparable studies for the major confounding factors 2) a wide range of markers of past and active HBV, HCV, and HDV infections and 3) absence of publication bias.
The facies of hepatic viruses circulating in Africa being specific to both genotypes and potential mutations, prospective longitudinal studies are required to determine the role of these parameters as biomarkers for the early diagnosis of the risk of progression to HCC 18,19,67. Further studies investigating the role of occult viral hepatitis and the role of the viral load and viral therapy in the progression to HCC are also required for African populations 11,68,69. In this review with only case-control studies including cases in the terminal phase of the disease with a poor prognosis, large-scale longitudinal studies on healthy populations with prospective follow-up and/or a systematic collection of health data could lead to the identification of more early predictors of progression to HCC, and thus hope to modify the high mortality of HCC cases recorded in Africa. HBV, the predominant etiological agent of HCC, is highly endemic in Africa. Due to the protective effect of HBV treatment on the risk of progression to HCC 70,71, it would be crucial to improve prevention through strong vaccination policies, diagnostic access, and appropriate HBV treatment. Improving access in Africa to new direct-acting HCV therapies could potentially be of importance in reducing the burden of HCC.
Taking into account a wide range of confounders, the findings of this review suggest that HBV/HCV coinfections and HBV, HCV, and HDV infections are associated with a high risk of the occurrence of HCC.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. 144: 1941–1953. pmid:30350310
2. Sung H, Ferlay J, Siegel RL (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
3. Boyle P, Levin B (2008) World Cancer Report 2008.
4. Maucort-Boulch D, de Martel C (2018) Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. 142: 2471–2477.
5. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM (2020) Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 8: e180–e190. pmid:31862245
6. Cho LY, Yang JJ, Ko K-P, Park B, Shin A, et al. (2011) Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: Systematic review and meta-analysis. International Journal of Cancer 128: 176–184. pmid:20232388
7. Donato F, Boffetta P, Puoti M (1998) A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. International Journal of Cancer 75: 347–354. pmid:9455792
8. Li L, Lan X (2016) Association between hepatitis B virus/hepatitis C virus infection and primary hepatocellular carcinoma risk: A meta-analysis based on the Chinese population. Journal of Cancer Research and Therapeutics 12: 284. pmid:28230038
9. Shi J, Zhu L, Liu S, Xie W-f (2005) A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. British Journal of Cancer 92: 607–612. pmid:15685242
10. Alfaiate D, Clément S, Gomes D, Goossens N, Negro F (2020) Chronic hepatitis D and hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Journal of Hepatology 73: 533–539. pmid:32151618
11. Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, et al. (2012) Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver International: Official Journal of the International Association for the Study of the Liver 32: 231–240. pmid:21745272
12. Bray F, Colombet M, Mery L, Piñeros M, Znaor A, et al. (2021) Cancer Incidence in Five Continents Volume XI.
13. Kew MC (2010) Hepatocellular carcinoma in African Blacks: Recent progress in etiology and pathogenesis. World J Hepatol 2: 65–73. pmid:21160975
14. Kramvis A, Kew MC (2007) Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res 37: S9–s19. pmid:17627641
15. Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 47 Suppl: S2–6. pmid:23632345
16. El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142: 1264–1273.e1261. pmid:22537432
17. An P-P, Feng L-N, Zhang X-X, Jin Q-L (2020) Association of interleukin-6 gene polymorphisms with the risk of hepatocellular carcinoma. Medicine 99. pmid:33327352
18. Liu S, Zhang H, Gu C, Yin J, He Y, et al. (2009) Associations Between Hepatitis B Virus Mutations and the Risk of Hepatocellular Carcinoma: A Meta-Analysis. JNCI: Journal of the National Cancer Institute 101: 1066–1082. pmid:19574418
19. Wong GL-H, Chan HL-Y, Yiu KK-L, Lai JW-Y, Chan VK-K, et al. (2013) Meta-analysis: the association of hepatitis B virus genotypes and hepatocellular carcinoma. Alimentary Pharmacology & Therapeutics 37: 517–526. pmid:23305043
20. Spearman CW, Sonderup MW (2015) Health disparities in liver disease in sub-Saharan Africa. Liver International: Official Journal of the International Association for the Study of the Liver 35: 2063–2071. pmid: 26053588
21. Spearman CW, Sonderup MW (2015) Health disparities in liver disease in sub-Saharan Africa. Liver Int 35: 2063–2071. pmid: 26053588
22. Kew MC (2013) Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol 12: 173–182. pmid:23396727
23. Bialecki ES, Di Bisceglie AM (2005) Diagnosis of hepatocellular carcinoma. HPB (Oxford) 7: 26–34. pmid:18333158
24. DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemporary Clinical Trials 45: 139–145. pmid:26343745
25. Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health 22: 153–160. pmid:31563865
26. Schwarzer G (2007) meta: An R package for meta-analysis. R News 7: 40–45.
27. Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 21: 1539– 1558. pmid:12111919
28. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ: British Medical Journal 315: 629–634. pmid:9310563
29. Kenmoe S, Bowo-Ngandji A, Kengne-Nde C, Ebogo-Belobo JT, Mbaga DS, Mahamat G, et al. Association between early viral LRTI and subsequent wheezing development, a meta-analysis and sensitivity analyses for studies comparable for confounding factors. van den Hoogen B, editor. PLoS ONE. 2021;16: e0249831. pmid:3385721 5
30. Amr S, Iarocci EA, Nasr GR, Saleh D, Blancato J, et al. (2014) Multiple pregnancies, hepatitis C, and risk for hepatocellular carcinoma in Egyptian women. BMC Cancer 14: 893. pmid:25432765
31. Bahri O, Ezzikouri S, Alaya-Bouafif NB, Iguer F, Feydi AE, et al. (2011) First multicenter study for risk factors for hepatocellular carcinoma development in North Africa. World J Hepatol 3: 24–30. pmid:21307984
32. Brown SE, Howard CR, Steward MW, Ajdukiewicz AB, Whittle HC (1984) Hepatitis B surface antigen containing immune complexes occur in seronegative hepatocellular carcinoma patients. Clinical and experimental immunology 55: 355–359. pmid:6199140
33. Cenac A, Develoux M, Lamothe F, Soubiran G, Vetter JM, et al. (1987) Delta superinfection in patients with chronic hepatitis, liver cirrhosis and hepatocellular carcinoma in a Sahelian area. Study of 112 cases versus 46 controls. Trans R Soc Trop Med Hyg 81: 994–997. pmid:2845609
34. Chin’ombe N, Chavhunduka E, Matarira HT (2009) Seroprevalence of HBV and HCV in primary hepatocellular carcinoma patients in Zimbabwe. Infect Agent Cancer 4: 15. pmid:19814789
35. Coursaget P, Leboulleux D, Le Cann P, Bao O, Coll-Seck AM (1992) Hepatitis C virus infection in cirrhosis and primary hepatocellular carcinoma in Senegal. Trans R Soc Trop Med Hyg 86: 552–553. pmid:1282278
36. Coursaget P, Maupas P, Goudeau A, Drucker J (1978) Incidence and significance of hepatitis B e antigen and antibody in postnecrotic cirrhosis and primary hepatocellular carcinoma. J Clin Microbiol 7: 394–395. pmid:211144
37. Cronberg S, Hansson BG, Thermos M, Moestrup T, Sow AM (1984) Hepatitis D (delta agent) in primary hepatocellular carcinoma and liver disease in Senegal. Liver 4: 275–279. pmid:6090857
38. Dhifallah I, Khedhiri M, Chouikha A, Kharroubi G, Hammami W, et al. (2020) Hepatitis viruses take advantage of traditional practices to increase the burden of hepatocellular carcinoma in Tunisia. Arch Virol 165: 33–42. pmid:31630275
39. Ezzat S, Abdel-Hamid M, Eissa SA, Mokhtar N, Labib NA, et al. (2005) Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health 208: 329–339. pmid:16217918
40. Gouas DA, Villar S, Ortiz-Cuaran S, Legros P, Ferro G, et al. (2012) TP53 R249S mutation, genetic variations in HBX and risk of hepatocellular carcinoma in The Gambia. Carcinogenesis 33: 1219–1224. pmid: 22759751
41. Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, et al. (2001) The role of hepatitis C in hepatocellular carcinoma— A case-control study among Egyptian patients. Journal of Clinical Gastroenterology 33: 123–126. pmid:11468438
42. Jaquet A, Tchounga B, Tanon A, Bagny A, Ekouevi DK, et al. (2018) Etiology of hepatocellular carcinoma in West Africa, a case-control study. Int J Cancer 143: 869–877. pmid:29569722
43. Kew MC, Desmyter J, Bradburne AF, Macnab GM (1979) Hepatitis B virus infection in southern African blacks with hepatocellular cancer. J Natl Cancer Inst 62: 517–520. pmid:84090
44. Kew MC, Houghton M, Choo QL, Kuo G (1990) Hepatitis C virus antibodies in southern African blacks with hepatocellular carcinoma. Lancet 335: 873–874. pmid: 1691422
45. Kew MC, Kassianides C, Hodkinson J, Coppin A, Paterson AC (1986) Hepatocellular carcinoma in urban born blacks: frequency and relation to hepatitis B virus infection. Br Med J (Clin Res Ed) 293: 1339–1341. pmid:3024771
46. Kirk GD, Lesi OA, Mendy M, Szymañska K, Whittle H, et al. (2005) 249(ser) TP53 mutation in plasma DNA, hepatitis B viral infection, and risk of hepatocellular carcinoma. Oncogene 24: 5858–5867. pmid:16007211
47. Larouzé B, Blumberg BS, London WT, Lustbader ED, Sankalé M, et al. (1977) Forecasting the development of primary hepatocellular carcinoma by the use of risk factors: studies in West Africa. J Natl Cancer Inst 58: 1557–1561. pmid:68119
48. Lightfoot K, Skelton M, Kew MC, Yu MC, Kedda MA, et al. (1997) Does hepatitis GB virus-C infection cause hepatocellular carcinoma in black Africans? Hepatology 26: 740–742. pmid:9303506
49. Mahale P, Aka P, Chen X, Pfeiffer RM, Liu P, et al. (2019) Hepatitis D virus infection, cirrhosis and hepatocellular carcinoma in The Gambia. J Viral Hepat 26: 738–749. pmid:30661282
50. Mak D, Babb de Villiers C, Chasela C, Urban MI, Kramvis A (2018) Analysis of risk factors associated with hepatocellular carcinoma in black South Africans: 2000– 2012. 13: e0196057. pmid:29718992
51. Mandishona E, MacPhail AP, Gordeuk VR, Kedda MA, Paterson AC, et al. (1998) Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatology 27: 1563–1566. pmid:9620327
52. Marchio A, Amougou Atsama M, Béré A, Komas NP, Noah Noah D, et al. (2018) Droplet digital PCR detects the high rate of TP53 R249S mutants in cell-free DNA of middle African patients with hepatocellular carcinoma. 18: 421–431. pmid:29749584
53. Mboto CI, Davies-Russell A, Fielder M, Jewell AP (2005) Hepatocellular Carcinoma in The Gambia and the role of Hepatitis B and Hepatitis C. J Hepatocell Carcinoma 2: 20. pmid:16202160
54. Mets T, Smitz J, Ngendahayo P, Sabbe L, Bigilimana I, et al. (1993) Hepatitis C virus infection in African patients with liver cirrhosis or primary hepatocellular carcinoma. Scand J Gastroenterol 28: 331–334. pmid: 8387695
55. Mohamed AE, Kew MC, Groeneveld HT (1992) Alcohol consumption as a risk factor for hepatocellular carcinoma in urban southern African blacks. Int J Cancer 51: 537–541. pmid:1318267
56. Montaser LM, Abbas OM, Saltah AM, Waked IA (2007) Circulating AFP mRNA as a Possible Indicator of Hematogenous Spread of HCC Cells: A Possible Association with HBV Infection. J Egypt Natl Canc Inst 19: 48–60. pmid:18839035
57. Nishioka K, Levin AG, Simons MJ (1975) Hepatitis B antigen, antigen subtypes, and hepatitis B antibody in normal subjects and patients with liver disease. Bull World Health Organ 52: 293–300. pmid:1084799
58. Ola SO, Akere A, Otegbayo JA, Odaibo GN, Olaleye DO, et al. (2012) Are patients with primary hepatocellular carcinoma infectious of hepatitis B, C, and D viruses? Afr J Med Med Sci 41: 187–191. pmid:23678655
59. Olubuyide IO, Aliyu B, Olalelye OA, Ola SO, Olawuyi F, et al. (1997) Hepatitis B and C virus and hepatocellular carcinoma. Trans R Soc Trop Med Hyg 91: 38–41. pmid: 9093625
60. Omer RE, Van’t Veer P, Kadaru AM, Kampman E, el Khidir IM, et al. (2001) The role of hepatitis B and hepatitis C viral infections in the incidence of hepatocellular carcinoma in Sudan. Trans R Soc Trop Med Hyg 95: 487–491. pmid:11706655
61. Schiefelbein E, Zekri AR, Newton DW, Soliman GA, Banerjee M, et al. (2012) Hepatitis C virus and other risk factors in hepatocellular carcinoma. Acta Virol 56: 235–240. pmid:23043603
62. Skelton M, Kew MC, Yu MC, Crookes RL, Swanevelder JP, et al. (2000) Transfusion-transmissible virus and hepatocellular carcinoma: a case-control study. J Viral Hepat 7: 230–234. pmid:10849266
63. Soliman AS, Hung CW, Tsodikov A, Seifeldin IA, Ramadan M, et al. (2010) Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol Int 4: 681–690. pmid:21286338
64. Tabor E, Gerety RJ, Vogel CL, Bayley AC, Anthony PP, et al. (1977) Hepatitis B virus infection and primary hepatocellular carcinoma. J Natl Cancer Inst 58: 1197– 1200. pmid:192895
65. Tswana SA, Moyo SR (1992) The interrelationship between HBV-markers and HIV antibodies in patients with hepatocellular carcinoma. J Med Virol 37: 161–164. pmid:1279108
66. Thi Vo T, Poovorawan K, Charoen P, Soonthornworasiri N, Nontprasert A, et al. (2019) Association between Hepatitis B Surface Antigen Levels and the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Infection: Systematic Review and Meta-Analysis. Asian Pacific journal of cancer prevention: APJCP 20: 2239–2246. pmid:31450890
67. Fang ZL, Sabin CA, Dong BQ, Ge LY, Wei SC, et al. (2008) HBV A1762T, G1764A mutations are a valuable biomarker for identifying a subset of male HBsAg carriers at extremely high risk of hepatocellular carcinoma: a prospective study. Am J Gastroenterol 103: 2254– 2262. pmid:18844615
68. Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, et al. (2007) Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis 195: 5–11. pmid:17152003
69. Truong BX, Yano Y, Seo Y, Phuong TM, Tanaka Y, et al. (2007) Variations in the core promoter/pre-core region in HBV genotype C in Japanese and Northern Vietnamese patients. J Med Virol 79: 1293–1304. pmid:17607788
70. Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, et al. (2015) Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol 62: 363–370. pmid:25195548
71. Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, et al. (2015) Impact of long-term tenofovir disoproxil fumarate on the incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 121: 3631–3638. pmid:26177866
Funding:
This project is part of the EDCTP2 Programme supported by the European Union under grant agreement TMA2019PF-2705. “The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.”
Competing interests
The authors have declared that no competing interests exist.
About the Authors
Donatien Serge Mbaga – Roles Conceptualization, Data curation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing
*E-mail: mbaga2015.mds@gmail. com (DSM); sebastien.kenmoe@ubuea. cm (SK)
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Sebastien Kenmoe – Roles Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
*E-mail: mbaga2015.mds@gmail.com (DSM); sebastien.kenmoe@ubuea.cm (SK)
Affiliation: Virology Department, Centre Pasteur of Cameroon, Yaoundé, Cameroon
Cyprien Kengne-Ndé – Roles Data curation, Formal analysis, Methodology, Validation, Writing – review & editing
Affiliation: Evaluation and Research Unit, National AIDS Control Committee, Yaoundé, Cameroon
Jean Thierry Ebogo-Belobo – Roles Data curation, Methodology, Validation, Writing – review & editing
Affiliation: Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Gadji Mahamat – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Joseph Rodrigue Foe-Essomba – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Mycobacteriology, Centre Pasteur of Cameroon, Yaoundé, Cameroon
Marie Amougou-Atsama – Roles Methodology, Validation, Writing – review & editing
Affiliation: Centre de Recherche sur les Maladies Émergentes et Re-Emergentes, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Serges Tchatchouang – Roles Methodology, Validation, Writing – review & editing
Affiliation: Bacteriology Department, Centre Pasteur of Cameroon, Yaoundé, Cameroon
Inès Nyebe – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Alfloditte Flore Feudjio – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Ginette Irma Kame-Ngasse – Roles Methodology, Validation, Writing – review & editing
Affiliation: Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Jeannette Nina Magoudjou-Pekam – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Lorraine K. M. Fokou – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Dowbiss Meta-Djomsi – Roles Methodology, Validation, Writing – review & editing
Affiliation: Centre de Recherche sur les Maladies Émergentes et Re-Emergentes, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Martin Maïdadi-Foudi – Roles Methodology, Validation, Writing – review & editing
Affiliation: Centre de Recherche sur les Maladies Émergentes et Re-Emergentes, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Sabine Aimee Touangnou-Chamda – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Audrey Gaelle Daha-Tchoffo – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Medical Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Abdel Aziz Selly-Ngaloumo – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Rachel Audrey Nayang-Mundo – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, Protestant University of Central Africa, Yaoundé, Cameroon
Jacqueline Félicité Yéngué – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Animals Biology and Physiology, The University of Yaounde I, Yaoundé, Cameroon
Jean Bosco Taya-Fokou – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Raoul Kenfack-Momo – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Biochemistry, The University of Yaounde I, Yaoundé, Cameroon
Efietngab Atembeh Noura – Roles Methodology, Validation, Writing – review & editing
Affiliation: Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies, Yaoundé, Cameroon
Cynthia Paola Demeni Emoh – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Hervé Raoul Tazokong – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Arnold Bowo-Ngandji – Roles Data curation, Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Carole Stéphanie Sake – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Etienne Atenguena Okobalemba – Roles Methodology, Validation, Writing – review & editing
Affiliation: Faculty of Medicine and Biomedical Science, The University of Yaoundé I, Yaoundé, Cameroon
Jacky Njiki Bikoi – Roles Methodology, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
Richard Njouom – Roles Methodology, Project administration, Validation, Writing – review & editing
Affiliation: Virology Department, Centre Pasteur of Cameroon, Yaoundé, Cameroon
Sara Honorine Riwom Essama – Roles Conceptualization, Methodology, Project administration, Validation, Writing – review & editing
Affiliation: Department of Microbiology, The University of Yaounde I, Yaoundé, Cameroon
CREDITS: Mbaga DS, Kenmoe S, Kengne-Ndé C, Ebogo-Belobo JT, Mahamat G, Foe-Essomba JR, et al. (2022) Hepatitis B, C, and D virus infections and risk of hepatocellular carcinoma in Africa: A meta-analysis including sensitivity analyses for studies comparable for confounders. PLoS ONE 17(1): e0262903. https://doi.org/10. 1371/journal.pone.0262903