Ane Bull Iversen1,2,3 , Rolf Ankerlund Blauenfeldt1 , Søren Paaske Johnsen4, Birgitte F Sandal5 , Bo Christensen2,3, Grethe Andersen1 and Morten Bondo Christensen2,3
1Department of Clinical Medicine – Neurology, Aarhus University, Aarhus N, Denmark
2Research Unit for General Practice, Aarhus C, Denmark
3Department of Public Health, Aarhus University, Aarhus C, Denmark
4Danish Center for Clinical Health Services Research, Department of Clinical Medicine, Aalborg University and Aalborg University Hospital, Aalborg, Denmark
5Department of Neurology, Regional Hospital of West Jutland, Holstebro, Denmark
Corresponding author:
Ane B Iversen, Department of Clinical Medicine – Neurology, Aarhus University, Palle Juul-Jensens Boulevard 165, Aarhus N DK-8200, Denmark.
Email: aneiversen@clin.au.dk
Abstract
Introduction
Only a minority of patients with acute ischaemic stroke receive reperfusion treatment, primarily due to prehospital delay. We aimed to investigate predictors of a primary contact to the emergency medical services, arrival at stroke centre within 3 h of symptom onset and initiation of reperfusion therapy in patients with acute stroke.
Patients and methods
We conducted a cross-sectional study of consecutive patients with acute ischaemic stroke, intracerebral haemorrhage or transient ischaemic attack. Structured interviews of patients and bystanders were performed and combined with clinical information from the Danish Stroke Registry. Eligible patients were aged ≥18 years and were independent in activities of daily living before the stroke.
Results
We included 435 patients. The presence of a bystander at symptom onset and knowledge of ≥2 core symptoms of stroke were associated with a primary emergency medical services contact. Higher stroke severity and patients or bystanders perceiving the situation as very serious were associated with a primary emergency medical services contact (ORpatients 2.10; 95% CI 1.12–3.95 and ORbystanders 22.60; 95% CI 4.98–102.67), <3 h from onset to arrival (ORpatients 3.01; 95% CI 1.46–6.21 and ORbystanders 4.44; 95% CI 1.37–14.39) and initiation of reperfusion therapy (ORpatients 3.08; 95% CI 1.23–7.75 and ORbystanders 4.70; 95% CI 1.14–19.5).
Conclusion: Having a bystander, knowledge of ≥2 core symptoms and understanding that a stroke is a serious event are associated with appropriate help-seeking behaviour, shorter prehospital delay and a higher chance of reperfusion therapy in acute stroke patients.
Keywords: Acute ischaemic stroke, intracerebral haemorrhage, stroke knowledge, help-seeking behaviour, prehospital delay, emergency medical services contact, reperfusion therapy
Introduction
Acute reperfusion therapies have radically changed acute stroke treatment, which has resulted in markedly improved prognosis and reduced mortality.1–4 However, the treatment must be started within 4.5–6 h (in some up to 24 h) from symptom onset for acute ischaemic stroke (AIS) and only a minority of AIS patients receive acute reperfusion treatment. This is primarily due to presentation outside the time window for intravenous thrombolysis and endovascular treatment at the stroke centre.5,6
Substantial efforts have been made to reduce both prehospital and in-hospital delays in stroke by educating physicians, staff at the emergency medical services (EMS) and the public, and by reorganising the stroke centres and the EMS.7,8 Patient delay is the main cause of prehospital delay.7,9 Many stroke patients do not know the symptoms of stroke or do not recognise their own symptoms as signs of a stroke. Others do not understand the seriousness of the situation and the importance of calling the EMS immediately, or they are unable to seek help unless they have a bystander.7,10 Some patients and bystanders take a ‘wait-and-see attitude’, or they contact the general practitioner (GP) or other healthcare professionals instead of the EMS, which may further extend the delay.6,9,11
Sparse knowledge exists on predictors of help-seeking behaviour and patient-related prehospital delay. We aimed to investigate patient- and bystander-related predictors of primary EMS contact, arrival at stroke centre within 3 h from symptom onset and initiation of reperfusion therapy in acute stroke patients, as these hallmarks are central in timely care for acute stroke.
Patients and methods
Design, sample and setting
We performed a cross-sectional study on consecutive patients with AIS, transient ischaemic attack (TIA) or intracerebral haemorrhage (ICH) admitted or seen in the neurovascular outpatient clinics in two-stroke centres in Central Denmark Region with 1.3 million inhabitants. The study took place between 28 January and 10 May 2018.
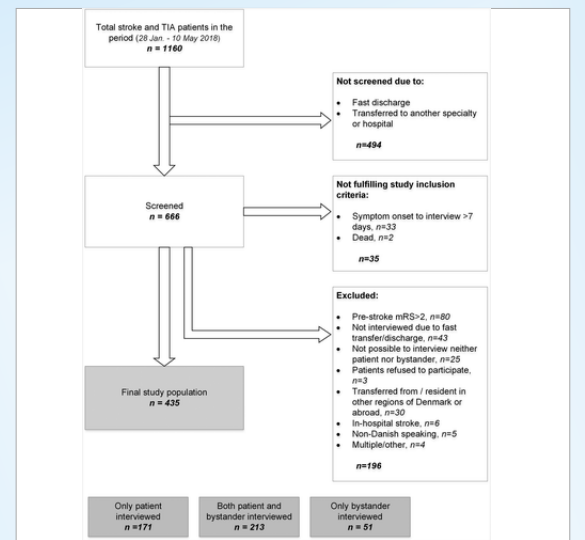
Eligible patients were ≥18 years, independent in activities of daily living (score ≤2 on the modified Rankin Scale) and ≤7 days from stroke onset to interview. Screening and inclusion of patients took place during normal working hours on weekdays. If a patient was admitted more than once during the study period, only the first admission was included. The exclusion criteria are shown in Figure 1.
A bystander was defined as the person who was with the patient at symptom onset, found the patient or was contacted/called by the patient. This was usually a family member or close relative, but it could also be, e.g. a friend or neighbour. As far as possible, we interviewed both the patient and the bystander (separately). If there was no bystander, or the bystander could not be reached, we only interviewed the patient. If the patient could not be interviewed due to severe aphasia, dysarthria, reduced consciousness, critical illness or mental impairment, we only interviewed the bystander. If there was no bystander in these cases, the patient was excluded.
The main outcome measures were primary EMS contact, arrival at stroke centre within 3 h after symptom onset and initiation of reperfusion therapy. A primary EMS contact meant that the first contact to a healthcare professional after the onset of stroke symptoms was directed to the EMS.
In Denmark, all citizens have free access to healthcare. GPs have a key position in the healthcare system as all citizens are listed with a GP and the GPs act as gatekeepers to the rest of the healthcare system. Each region has an out-of-hours primary care (OOH-PC) service that covers the entire region. The citizens should call their own GP in the daytime and the OOH-PC service in the evenings and nights if they need medical help. If the condition is so severe that immediate action is needed, they should call the EMS.12 However, it can be difficult for a patient or bystander to choose the correct way into the healthcare system, especially in an acute situation. This might influence the type of help received and how fast such help is initiated.
Approximately 90% of all known stroke patients are seen at a stroke centre.13,14
The study protocol was assessed as a quality improvement study and patient consent was consequently not needed. A statement from the Central Denmark Region Committee on Health Research Ethics concluded that the study could be conducted without approval from the Committee.
Data collection
Based on detailed pilot interviews with 30 consecutive stroke patients admitted to the Department of Neurology at Aarhus University Hospital, we developed a structured interview.
The interviews took place as soon as possible after admission and at the latest within seven days from symptom onset, otherwise, the patient was excluded. Bystanders were interviewed when they visited the patient at the stroke unit, otherwise by phone.
The first part of the interview assessed both the patient’s and the bystander’s knowledge of stroke and core symptoms of stroke, their education and prior experience with stroke (private or professional). We asked them to disregard any knowledge received after symptom onset. Core symptoms of stroke were defined as facial palsy, palsy of extremities and aphasia/ dysarthria. All are common stroke symptoms and frequently used in stroke campaigns.15–17
The second part of the interview explored the course of events on the day of symptom onset, including exact time of symptom onset, presence of/ contact to a bystander, response to symptoms (e.g. if patient/bystander took no action or called for help). If they chose to seek help, we asked who was contacted and for the exact time of contact.
Interviews contained both closed- and open-ended questions and no alternative response options were given. The interviewers were research nurses, research physicians and a medical student. All were trained in performing structured interviews and followed a written guide. Responses to the questions were entered directly in the electronic case report form using a tablet and afterwards uploaded to the database (REDCap).18,19 The interviews can be found in the supplemental material.
Data on demographics, clinical characteristics and treatment received were obtained from the medical record and the Danish Stroke Registry.20
Variables
The dependent variables included primary EMS contact, arrival at stroke centre within 3 h after symptom onset and initiation of reperfusion therapy. The independent clinically relevant variables included patient age, patient sex, stroke severity (measured by the Scandinavian Stroke Scale (SSS),21 histories of stroke or TIA in the patient and presence of/call for a bystander. The independent variables on education, healthcare professional background, personal experience with stroke from family or friends, knowledge of stroke core symptoms and perceived seriousness of the situation at onset were taken from the interview of either patient or bystander.
The SSS is a validated measure of neurologic impairment and ranges from 0 to 58, a higher score indicating less severe stroke. It can easily be converted to the more known National Institutes of Health Stroke Scale.21–23 In the analyses, the score was categorised into mild (43–58), moderate (26–42) and severe (0–25) stroke.22
Statistical analysis
We compared normally distributed data by pairwise comparison of means with equal variance and non-normal distributed data by Wilcoxon rank-sum test or Kruskal–Wallis test as appropriate. Univariate and multivariable logistic regression analyses were performed to examine the association between the independent and dependent variables. We conducted the analyses on the patient population and the bystander population separately, using independent variables from the patient interview and the bystander interview, respectively.
A p-value <0.05 was considered statistically significant. Data management and statistics were performed using Stata 15.1 software (StataCorp, College Station, TX, USA).
Results
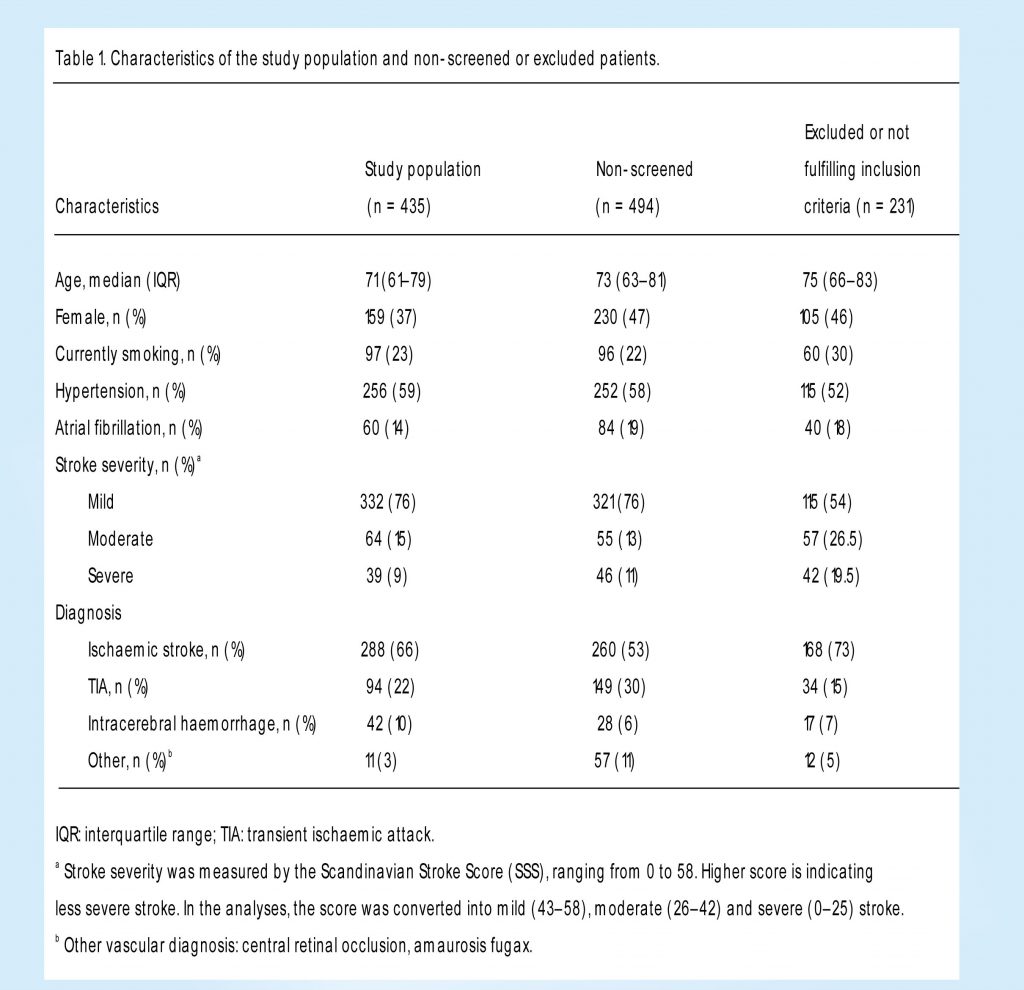
Out of 1160 patients with stroke or TIA admitted in the study period, 666 patients were screened. Of the screened patients, 35 did not fulfil study inclusion criteria, and 196 were excluded. The final study population included 435 patients (Figure 1) of whom 80% had a bystander. We performed 384 interviews with patients and 264 interviews with bystanders. The median time from admission to interview was one day (interquartile range (IQR) 1–1).
The median patient age was 71 years (IQR 61–79). Only 37% were female, which was significantly lower than in the non-screened population. Minor stroke was found in 76% of the patients, 15% had a moderate stroke and 9% a severe stroke. This was very similar to the non-screened population (Table 1).
Knowledge, perceived seriousness and help-seeking behaviour
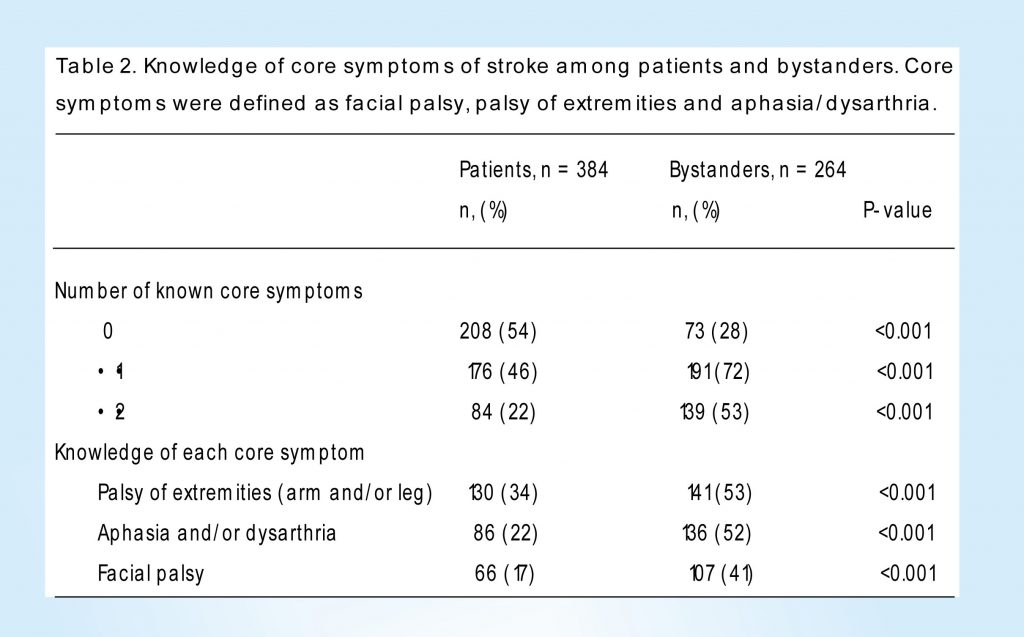
Knowledge of core symptoms was significantly lower in patients than in bystanders. Of patients, 54% could not mention any stroke core symptom, 46% could mention at least one core symptom and 22% could mention at least two symptoms. In bystanders, the corresponding percentages were 28, 72 and 53%, respectively (Table 2).
Patients generally perceived the situation at symptom onset as less serious than bystanders did. Of patients, 62% assessed the situation as not serious or less serious, 16% as moderately serious and 22% as very serious. For bystanders, the corresponding percentages were 19, 23 and 58%, respectively. When comparing perceived seriousness with actual stroke severity (SSS score), we found that bystanders’ assessment of seriousness more closely resembled the professional stroke centre assessment of seriousness than the patients’ assessment (data not shown).
A primary EMS call was made for 29% of the patients. In 28% the GP was the first to be contacted, in 21% the OOH-PC, in 14% family or friends outside the home and in 9% other healthcare professionals or unknown.
Primary EMS contact
In the patient population, higher age (80–99 years) was associated with less chance of a primary EMS contact compared to the youngest age group (18–59 years). Having a moderate or severe stroke, having a bystander present, knowledge of ≥2 core symptoms and perceiving the situation as very serious were associated with a primary EMS contact (unadjusted analyses) (Table 3).
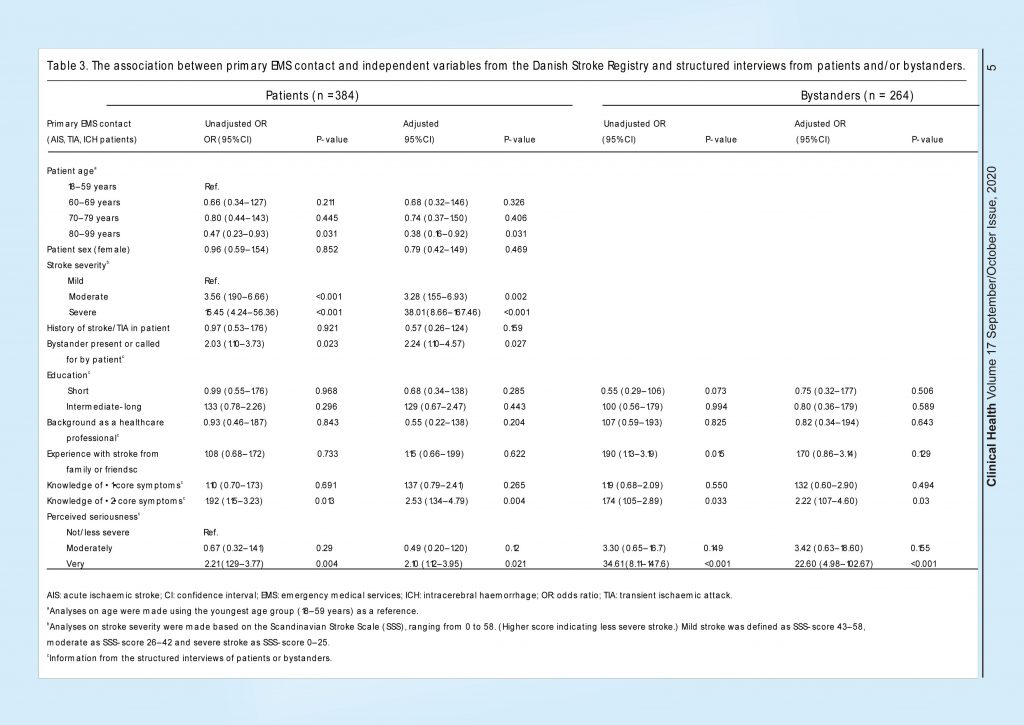 In the multivariable analysis, these associations were confirmed; higher age (80–99 years) was associated with less chance of a primary EMS contact compared to the youngest age group. Having a moderate or severe stroke, having a bystander present (adjusted OR 2.24; 95% CI 1.10–4.57), knowledge of ≥2 core symptoms (adjusted OR 2.53; 95% CI 1.34–4.79) and perceiving the situation as very serious (adjusted OR 2.10; 95% CI 1.12–3.95) were associated with a primary EMS contact. In the bystander population, we also found that a moderate or severe stroke inpatient, bystanders knowledge of ≥2 core symptoms (adjusted OR 2.22; 95% CI 1.07–4.60) and bystanders perceiving the situation as very serious (adjusted OR 22.60; 95% CI 4.98–102.67) were associated with a primary EMS contact.
In the multivariable analysis, these associations were confirmed; higher age (80–99 years) was associated with less chance of a primary EMS contact compared to the youngest age group. Having a moderate or severe stroke, having a bystander present (adjusted OR 2.24; 95% CI 1.10–4.57), knowledge of ≥2 core symptoms (adjusted OR 2.53; 95% CI 1.34–4.79) and perceiving the situation as very serious (adjusted OR 2.10; 95% CI 1.12–3.95) were associated with a primary EMS contact. In the bystander population, we also found that a moderate or severe stroke inpatient, bystanders knowledge of ≥2 core symptoms (adjusted OR 2.22; 95% CI 1.07–4.60) and bystanders perceiving the situation as very serious (adjusted OR 22.60; 95% CI 4.98–102.67) were associated with a primary EMS contact.
Arrival at stroke centre within 3 h
The median time from symptom onset to arrival at a stroke centre was 6.4 h (IQR 2.0–23.0 h) and 30% of the patients arrived within 3 h. Analyses of predictors for arrival at stroke centre within 3 h were performed on AIS and ICH patients only, as TIA patients do not always have to be triaged for acute arrival at the stroke centre.
In the patient population, having a moderate or severe stroke and perceiving the situation as very serious (adjusted OR 3.01; 95% CI 1.46–6.21) were associated with arrival within 3 h (Table 4). In the bystander population, severe stroke inpatient and bystander perceiving the situation as very serious were also associated with patient arrival within 3 h (adjusted OR 4.44; 95% CI 1.37–14.39).
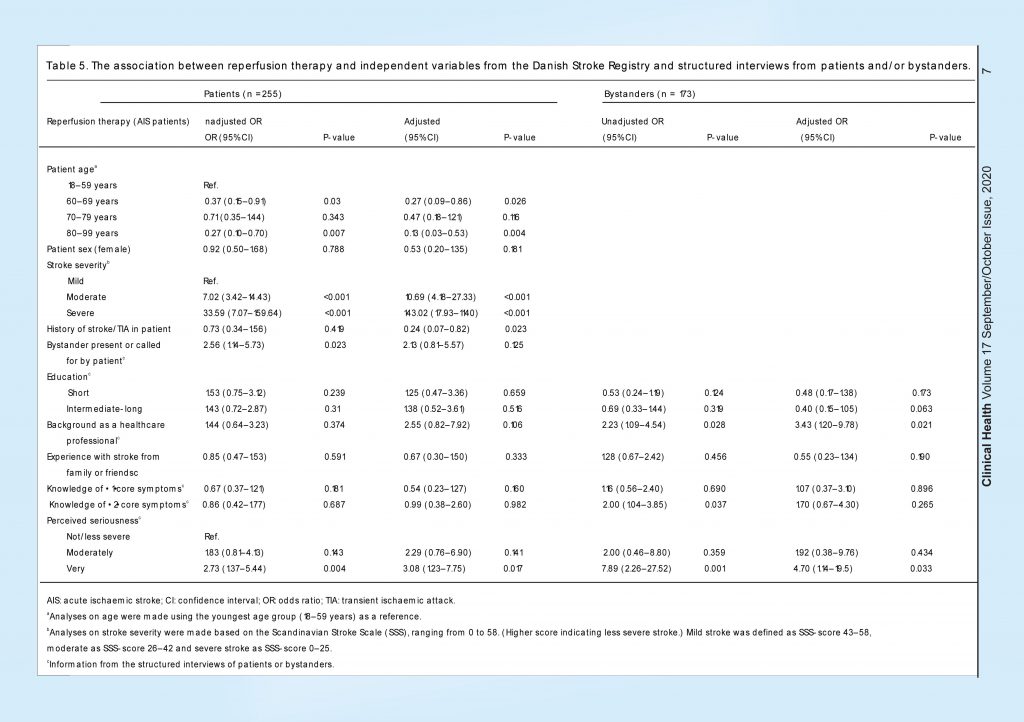
A total of 76 (26%) of the AIS patients received reperfusion therapy. Analyses of predictors for reperfusion therapy were performed on the AIS population only (Table 5). In the patient population, higher age (80–99 years) was associated with less chance of receiving reperfusion therapy compared to the youngest age group (18–59 years). Moderate and severe stroke and perceiving the situation as very serious (adjusted OR 3.08; 95% CI 1.23–7.75) were associated with a higher chance of receiving reperfusion therapy.
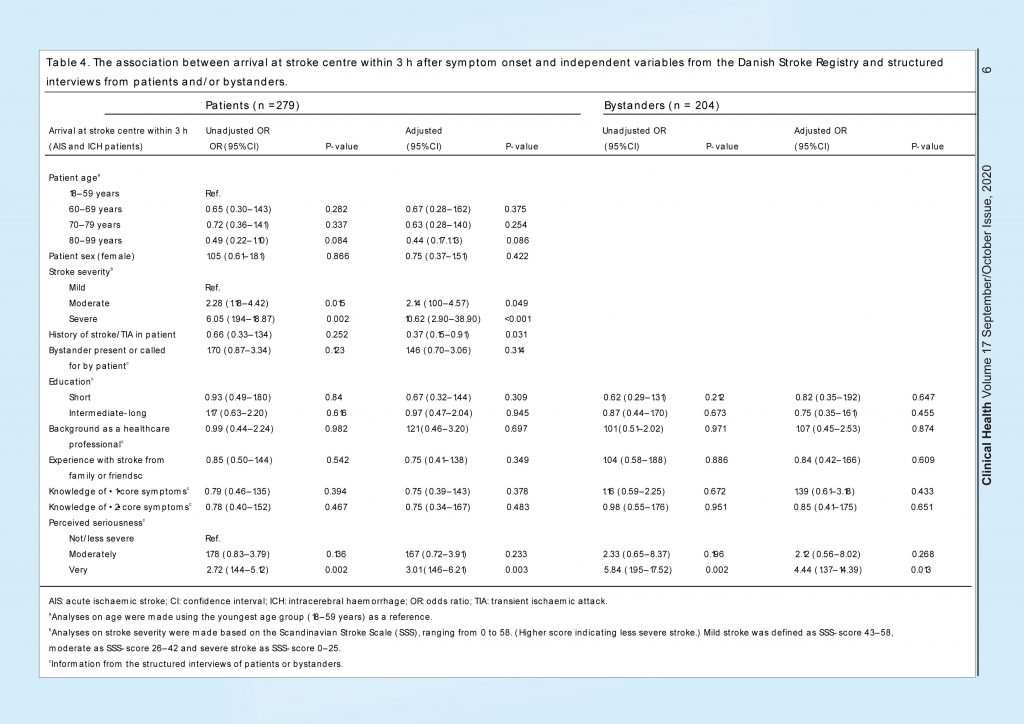 In the bystander population, we found the same associations for higher patient age and stroke severity. Bystanders’ background as a healthcare professional (adjusted OR 3.43; 95% CI 1.20–9.78) and bystanders perceiving the situation as very serious (adjusted OR 4.70; 95% CI 1.14–19.5) were associated with a higher chance for the patient to receive reperfusion therapy.
In the bystander population, we found the same associations for higher patient age and stroke severity. Bystanders’ background as a healthcare professional (adjusted OR 3.43; 95% CI 1.20–9.78) and bystanders perceiving the situation as very serious (adjusted OR 4.70; 95% CI 1.14–19.5) were associated with a higher chance for the patient to receive reperfusion therapy.
We found no significant association with female sex, intermediate to long education or experience with stroke from family or friends with any of the main outcomes.
Discussion
Compared to bystanders, stroke patients perceived the situation at symptom onset as less serious and had less knowledge of the core symptoms. A primary EMS contact was made for less than one in three patients. For half of the patients, either the GP or OOH-PC was the first to be contacted. Higher patient age was associated with a reduced chance of a primary EMS contact and initiation of reperfusion therapy. Having a moderate or severe stroke and patients and/or bystanders also perceiving the situation as very serious were associated with a primary EMS contact, arrival at stroke centre within 3 h from symptom onset and initiation of reperfusion therapy. Having a bystander and knowledge of ≥ 2 core symptoms were moreover associated with a primary EMS contact. A powerful predictor for all outcomes, together with high stroke severity, was having a bystander perceive the situation as very serious.
To our knowledge, this is the first study to systematically collect detailed information on knowledge of stroke symptoms, previous experience with stroke and perceived seriousness of the situation in both patients and bystanders, combined with information on help-seeking behaviour, treatment and medical history. Acute cognitive impairment frequently occurs after acute stroke and even TIA.24,25 Hence, understanding the situation surrounding an acute stroke may be improved by interviewing the bystanders. Therefore, including bystanders is important to fully understand patient- and bystander-related predictors of delay. We included a large population of consecutive acute stroke patients and bystanders over a short period. The short time from admission to interview may limit the risk of recall bias.
However, our study also has limitations. The number of screened and included patients was lower than the total number of stroke and TIA patients in the period. Screening and inclusion of patients took place during normal working hours on weekdays. However, inclusion was omitted on some weekdays (randomly distributed) due to a lack of interviewers. Some patients with TIA or minor stroke spent only a few hours in the outpatient clinic, and other patients were discharged or transferred after one day. Although we found some differences between screened and non-screened patients, only a minor difference was seen in stroke severity between the groups. The risk of selection bias was considered low. In the interview, we asked questions on stroke knowledge and perceived seriousness at symptom onset. We informed the patient and bystander to exclude any knowledge received from any healthcare provider or acquired from their own experience after symptom onset. This could be difficult, and knowledge obtained after symptom onset could have affected the answers to some extent. The patients’ responses could also have been influenced by acute cognitive impairment. However, interviewed patients were able to answer all questions and remember what happened from symptom onset until admission.
Including bystanders seems essential as they often are the ones to take action and call for help.26,27 Shah et al.26 have previously reported that only 31% of the patients recognised the need to seek help, whereas 59% of the bystanders did. This corresponds with our findings on the perceived seriousness of the situation in patients and bystanders. In line with our study, some studies have found that bystander presence increases the use of EMS,27–29 and others have also found an association to reduced prehospital delay.7,10,29 However, the role of the bystander and the patient’s type of social network may play an important role, as smaller social networks (e.g. with only one family member) may maintain the patient in a ‘wait-and-see’-behaviour.30
Two studies by Faiz et al.9,31 explored the reasons for prehospital delay and ‘decision delay’ in acute stroke, but did not include interpretation of seriousness. They collected information on the patients’ knowledge of stroke, but no association was found to delay when comparing patients with and without previous stroke knowledge.
Some studies evaluating educational stroke campaigns have questioned whether improved knowledge can be transformed into symptom recognition and correct help-seeking behaviour at symptom onset,32,33 and previous campaigns might have focussed too narrowly on stroke knowledge. Some studies have reported reduced prehospital delay and an increased number of patients treated with reperfusion therapy. However, methodological weaknesses make it difficult to generalise.5,7,34 Campaigns are shown to be most effective when the message is simple, repeated several times in different types of media, and both public and healthcare professionals are target groups.33,35
Understanding stroke as a severe and potentially life-threatening disease requiring urgent action is essential, as this understanding seems to be independently associated with correct help-seeking behaviour, timely arrival at stroke centre and higher reperfusion therapy rates in stroke patients. When designing future educational campaigns or sending other information on stroke to the public or to healthcare professionals, the severity and urgency of stroke must have a strong focus, and the important role of bystanders should be pinpointed. Bystanders could have a pivotal role to act, seek help and ultimately improve patient outcomes, as seen for out-of-hospital cardiac arrest.36 Health authorities and GP organisations should focus on high-quality triage of stroke patients, as GPs and OOH-PC are often the first to be contacted.
Conclusion
Knowledge of core symptoms of stroke is important, but this knowledge cannot stand alone. Understanding that a stroke is a serious and potentially life-threatening event should be underlined. Moreover, it should be stressed that patients seem to depend on bystanders to take prompt action and call the EMS. This may reduce prehospital delay, increase reperfusion therapy rates and ultimately ensure a better prognosis for stroke patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Tryg Fonden, Committee for Quality Improvement and Continuing Education in general practice in the Central Denmark Region (grant number 1-30-72-185-17) and the Multipractice Study Committee of the Danish College of General Practitioners (grant number 2017-00020).
Ethical approval
Ethical approval for this study was waived by the Central Denmark Region Committee on Health Research Ethics, 22 December 2017, ID 1-10-72-168-17. They concluded that the study could be conducted without approval from the Committee.
Informed consent
Verbal informed consent was obtained from all subjects before the study. Written informed consent was not obtained because the study protocol was assessed as a quality improvement study and formal consent was consequently not needed.
Guarantor
MBC.
Contributorship
ABI, RAB, SPJ, BS, BC, GA and MBC were involved in the study design and revision of the manuscript. ABI wrote the study protocol, gained approvals, collected data and wrote the manuscript. ABI and RAB performed the statistical analyses. All authors read and approved the final manuscript.
Acknowledgements
We thank the participants and their families for their participation in the study.
We thank study nurses Rikke Bay Thomsen1, Schiela Jensen1, Rena Hoffmann2, Kristina Eiskjær Sørensen1 and medical student Maria Kjølhede Sørensen1 for helping with data collection, Andreas Gammelgaard Damsbo, MD1 for technical support with the eCRF and statistician Henrik Schou Pedersen3 for advice on the statistical analyses.
1Department of Clinical Medicine – Neurology, Aarhus University, Palle Juul-Jensens Boulevard 165, DK-8200, Aarhus N, Denmark
2Department of Neurology, Regional Hospital of West Jutland, Laegaardsvej 12, DK-7500 Holstebro, Denmark
3Research Unit for General Practice, Bartholins Allé 2, DK-8000 Aarhus C, Denmark
References
1. Hacke, W, Kaste, M, Bluhmki, E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. Google Scholar | Crossref | Medline | ISI
2. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1588. Google Scholar | Crossref | Medline | ISI
3. Berkhemer, OA, Fransen, PSS, Beumer, D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. Google Scholar | Crossref | Medline | ISI
4. Lopes, DK, Kirmani, JF, Pereira, VM, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2017; 378: 11–21. Google Scholar
5. Fassbender, K, Balucani, C, Walter, S, et al. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol 2013; 12: 585–596. Google Scholar | Crossref | Medline | ISI
6. Thijs, V, Lemmens, R, Bouckaert, M. Reducing prehospital delay in acute stroke. Nat Rev Neurol 2009; 5: 477–483. Google Scholar | Crossref
7. Teuschl, Y, Brainin, M. Stroke education: discrepancies among factors influencing prehospital delay and stroke knowledge. Int J Stroke 2010; 5: 187–208. Google Scholar | SAGE Journals | ISI
8. Fonarow, GC, Zhao, X, Smith, EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014; 311: 1632–1640. Google Scholar | Crossref | Medline | ISI
9. Faiz, KW, Sundseth, A, Thommessen, B, et al. Prehospital delay in acute stroke and TIA. Emerg Med J 2013; 30: 669–674.Google Scholar | Crossref | Medline
10. Mellon, L, Doyle, F, Williams, D, et al. Patient behaviour at the time of stroke onset: a cross-sectional survey of patient response to stroke symptoms. Emerg Med J 2016; 33: 396–402. Google Scholar| Crossref
11. Soomann, M, Vibo, R, Kõrv, J. Acute stroke: why do some patients arrive in time and others do not? Eur J Emerg Med 2015; 22: 285–287. Google Scholar| Crossref
12. Ministry of Health. Healthcare in Denmark, https://www.sum.dk/ English/Healthcare-in-Denmark-an overview.aspx (accessed 7 February 2020). Google Scholar
13. Hastrup, S, Johnsen, SP, Terkelsen, T, et al. Effects of centralizing acute stroke services: a prospective cohort study. Neurology 2018; 91: e236–e248. Google Scholar | Crossref
14. Danish Stroke Registry, annual report 2018, https://www. sundhed.dk/ content/cms/69/4669_dap_aarsrapport2018_24062019final.pdf Final version: 24th June 2019. Author names: Dorthe Damgaard and Peter Vögele (In reference 14: Damgaard D, Vögele P). Google Scholar
15. Trobbiani, K, Freeman, K, Arango, M, et al. Comparison of stroke warning sign campaigns in Australia, England, and Canada. Int J Stroke 2013; 8: 28–31. Google Scholar | SAGE Journals | ISI
16. Nordanstig, A, Asplund, K, Norrving, B, et al. Impact of the Swedish National Stroke Campaign on stroke awareness. Acta Neurol Scand 2017; 136: 345–351. Google Scholar | Crossref | Medline
17. Bray, JE, O’Connell, B, Gilligan, A, et al. Is FAST stroke smart? Do the content and language used in awareness campaigns describe the experience of stroke symptoms? Int J Stroke 2010; 5: 440–446. Google Scholar | SAGE Journals | ISI
18. Harris, PA, Taylor, R, Thielke, R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. Google Scholar | Crossref | Medline | ISI
19. Harris, PA, Taylor, R, Minor, BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208, 1–10. Google Scholar | Crossref
20. Johnsen, S, Ingeman, A, Holmager Hunborg, H, et al. The Danish Stroke Registry. Clin Epidemiol 2016; 8: 697–702. Google Scholar | Crossref | Medline | ISI
21. Ali, K, Cheek, E, Sills, S, et al. Development of a conversion factor to facilitate comparison of National Institute of Health Stroke Scale scores with Scandinavian Stroke Scale scores. Cerebrovasc Dis 2007; 24: 509–515. Google Scholar | Crossref
22. Govan, L, Langhorne, P, Weir, CJ. Categorizing stroke prognosis using different stroke scales. Stroke 2009; 40: 3396–3399. Google Scholar| Crossref| Medline |ISI
23. Askim, T, Bernhardt, J, Churilov, L, et al. The Scandinavian Stroke Scale is equally as good as the National Institutes of Health Stroke Scale in identifying a 3-month outcome. J Rehabil Med 2016; 48: 909–912. Google Scholar | Crossref
24.Halevi DR, Bursaw AW, Karamchandani RR et al. Cognitive deficits in acute mild ischemic stroke and TIA and effects of rt-PA. Ann Clin Transl Neurol 2019; 6: 466–474. Google Scholar
25. Pinter, D, Enzinger, C, Gattringer, T, et al. Prevalence and short-term changes of cognitive dysfunction in young ischaemic stroke patients. Eur J Neurol 2019; 26: 727–732. Google Scholar | Crossref
26. Shah, M, Makinde, KA, Thomas, P. Cognitive and behavioural aspects affecting early referral of acute stroke patients to the hospital. J Stroke Cerebrovasc Dis 2007; 16: 71–76. Google Scholar | Crossref | Medline
27. Mosley, I, Nicol, M, Donnan, G, et al. Stroke symptoms and the decision to call for an ambulance. Stroke 2007; 38: 361–366. Google Scholar | Crossref | Medline | ISI
28. Ashkenazi, L, Toledano, R, Novack, V, et al. Emergency department companions of stroke patients. Medicine 2015; 94: e520. Google Scholar | Crossref
29. García Ruiz, R, Silva Fernández, J, García Ruiz, RM, et al. Response to symptoms and prehospital delay in stroke patients. Is it time to reconsider stroke awareness campaigns? J Stroke Cerebrovasc Dis 2018; 27: 625–632. Google Scholar | Crossref
30. Dhand, A, Luke, D, Lang, C, et al. Social networks and risk of delayed hospital arrival after acute stroke. Nat Commun 2019; 10: 1–8. Google Scholar | Crossref
31. Faiz, KW, Sundseth, A, Thommessen, B, et al. Factors related to decision delay in acute stroke. J Stroke Cerebrovasc Dis 2014; 23: 534. Google Scholar|Crossref| Medline |ISI
32. Zock, E, Kerkhoff, H, Kleyweg, RP, et al. Help-seeking behaviour and onset-to-alarm time in patients with acute stroke: sub-study of the preventive antibiotics in stroke study. BMC Neurol 2016; 16: 1–8. Google Scholar | Crossref
33. Lecouturier, J, Rodgers, H, Murtagh, MJ, et al. Systematic review of mass media interventions designed to improve public recognition of stroke symptoms, emergency response and early treatment. BMC Public Health 2010; 10: 784. Google Scholar|Crossref| Medline |ISI
34.Kobayashi, A, Czlonkowska, A, Ford, GA, et al. European Academy of Neurology and European Stroke Organization consensus statement and practical guidance for pre-hospital management of stroke. Eur J Neurol 2018; 25: 425–433. Google Scholar| Crossref
35. Rasura, M, Baldereschi, M, Di Carlo, A, et al. Effectiveness of public stroke educational interventions: a review. Eur J Neurol 2014; 21: 11–20. Google Scholar | Crossref | Medline
36. Kragholm, K, Wissenberg, M, Mortensen, RN, et al. Bystander efforts and 1-year outcomes in out-of-hospital cardiac arrest. N Engl J Med 2017; 376: 1737–1747.
Google Scholar Crossref | Medline
Credits: Iversen, A. B., Blauenfeldt, R. A., Johnsen, S. P., Sandal, B. F., Christensen, B., Andersen, G., & Christensen, M. B. (2020). Understanding the seriousness of a stroke is essential for appropriate help-seeking and early arrival at a stroke centre: A cross-sectional study of stroke patients and their bystanders. European Stroke Journal. https://doi.org/10.1177/2396987320945834