Julie Huynh 1,2,*, Yara-Natalie Abo 3,4, Karen du Preez 5, Regan Solomons 6, Kelly E Dooley 7 and James A Seddon 5,8
1. Oxford University Clinical Research Unit, Centre for Tropical Medicine, Hospital for Tropical Diseases, Ho Chi Minh City 700000, Vietnam
2. Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, Oxford University, Oxford OX1 2JD, UK
3. Infectious Diseases Unit, The Royal Children’s Hospital Melbourne, Parkville, VIC 3052, Australia
4. Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, VIC 3052, Australia
5. Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town 7600, South Africa
6. Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town 7600, South Africa
7. Department of Medicine–Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, MD 21218, USA
8. Department of Infectious Diseases, Imperial College London, London SW7 2BX, UK
*
Author to whom correspondence should be addressed.
Academic Editor: Leonardo A. Sechi
Abstract
Tuberculous meningitis disproportionately affects young children. As the most devastating form of tuberculosis, it is associated with unacceptably high rates of mortality and morbidity even if treated. Challenging to diagnose and treat, tuberculous meningitis commonly causes long-term neurodisability in those who do survive. There remains an urgent need for strengthened surveillance, improved rapid diagnostics technology, optimised anti-tuberculosis drug therapy, investigation of new host-directed therapy, and further research on long-term functional and neurodevelopmental outcomes to allow targeted intervention. This review focuses on the neglected field of paediatric tuberculous meningitis and bridges current clinical gaps with research questions to improve outcomes from this crippling disease.
Keywords: tuberculous meningitis; TBM; disseminated; central nervous system
1. Introduction
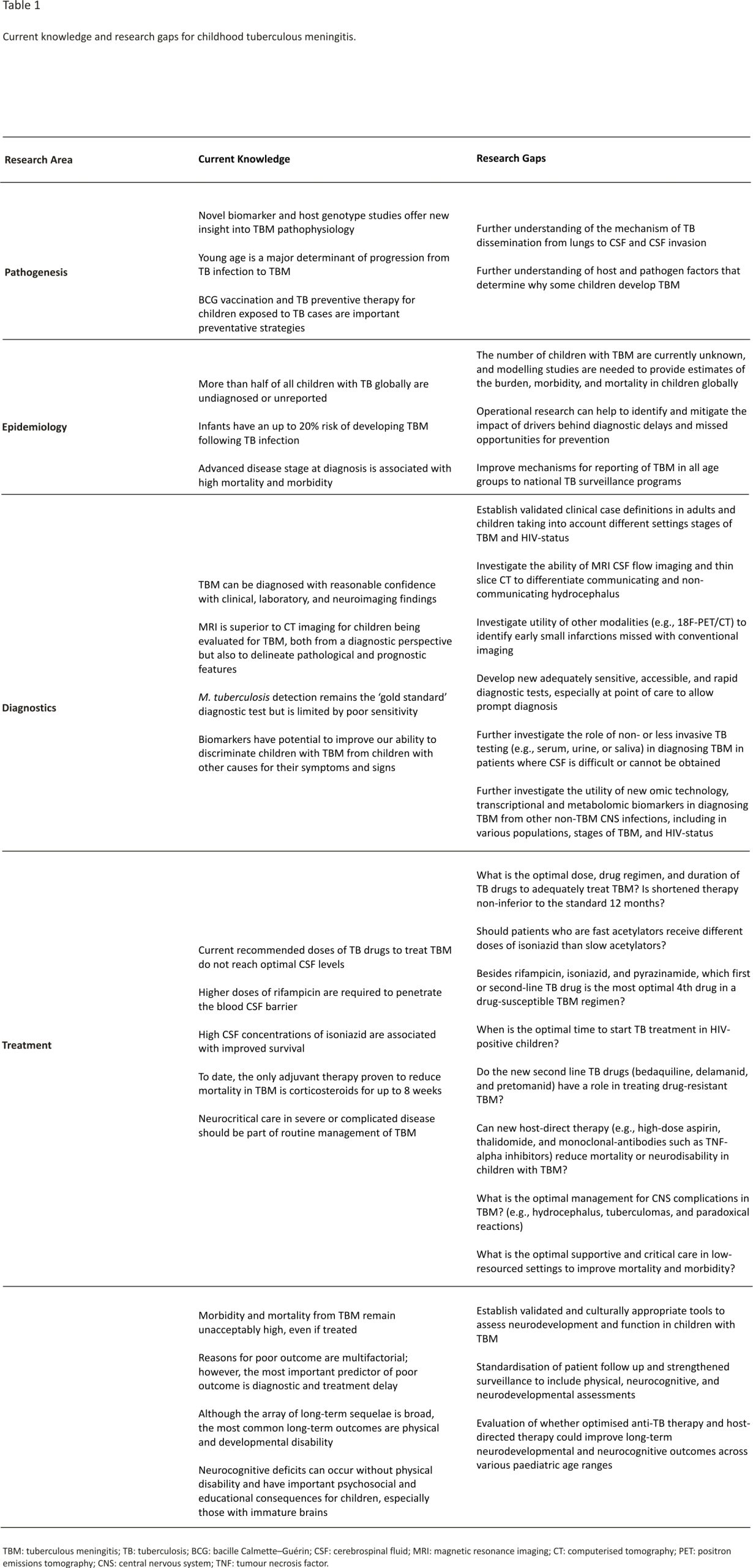
Young children and individuals living with HIV are at high risk of progressing to tuberculosis (TB) disease following TB infection and are at elevated risk of progressing to severe forms of disease such as disseminated TB and tuberculous meningitis (TBM) 1. TBM is the most devastating form of TB and is associated with high mortality and morbidity. Untreated, all children will die 2. Even if diagnosed and treated, 20% of children die and of those surviving over half have neurological disability 3. TBM in children, therefore, merits special consideration. In this article, we review the natural history and pathogenesis of TBM in children, the epidemiology of the disease, approaches to diagnosis, developments in treatment and considerations for long-term prognosis. We present recent research findings and areas that require prioritised future investigation (Table 1).
2. Natural History and Pathogenesis
After inhalation of M. tuberculosis-containing droplets, bacilli may deposit into the terminal alveoli. If they overcome the structural defences and innate immune response, an inflammatory process involving cytokine release, granuloma formation, and primary infection will ensue in the lungs. During this process, bacteraemia can occur where bacilli are filtered into draining lymph nodes and then onto the systemic circulation and distant sites, including the central nervous system (CNS) 4. M. tuberculosis may then invade the blood-brain barrier via (1) rearrangement of actin; (2) M. tuberculosis virulence factor(s) interacting with extracellular brain endothelium factors to facilitate bacillary endothelial adhesion; or (3) the ‘Trojan horse’ mechanism via infected macrophages and neutrophils 5,6,7,8. Once bacilli have gained access to the brain, a subcortical or meningeal ‘Rich focus’ is formed via activation of microglial cells and astrocytes 9,10. When this Rich focus is activated (rapidly in the context of military TB, as demonstrated in young children 10, or months to years later), M. tuberculosis is released into the subarachnoid space triggering a T-cell mediated inflammatory cascade including induction of pro-and anti-inflammatory cytokines such as tumour necrosis factor-alpha, interferon-gamma, interleukin (IL) 1b, IL-6, IL-8, and IL-10 4. The consequent formation of exudate envelopes arteries and nerves, disrupting cerebrospinal fluid (CSF) flow and contributing to the development of vasculitis in the vessels of the Circle of Willis, the vertebrobasilar system, and the perforating branches of the middle cerebral artery. Resultant hydrocephalus and infarct contribute to the clinical presentation in TBM 11.
Whether the mycobacteria are contained or cause clinical disease, and the extent of clinical disease, is determined by an interplay of host immune response and M. tuberculosis virulence factors, however, our understanding of these processes remains incomplete. Studies in paediatric 12,13,14 and adult TBM 13 demonstrate associations between immune mediators and clinical outcomes and suggest that a disequilibrium of pro-and anti-inflammatory cytokines underlies the severity and course of TBM. This balance can be regulated by Leukotriene A4 Hydroxylase (LTA4H); a gene that encodes an enzyme which influences the balance of pro-and anti-inflammatory eicosanoids seen in intracerebral inflammation. Variations of the LTA4H genotype may contribute to the heterogeneity of the inflammatory response and outcomes in TBM 15 Studies are ongoing to further examine the role of the LTA4H genotype on the immunoinflammatory response and the possibility of personalising adjunctive anti-inflammatory therapy based on host genotype 13 Clues to further understanding the biology of cerebral injury in TBM have come from biomarker signatures in TBM-infected children presenting with stroke 1,14 and transcriptional profiles demonstrating compartmentalisation of the immune response within the CNS (ventricular vs. lumbar CSF) 16.
A review of the natural history of childhood intrathoracic tuberculosis in the pre-chemotherapy era found that young age (<2 years of age) was the major determinant of progression from TB infection to disease; pulmonary disease developed in 30–40% and TBM or miliary disease in 10–20% of infants, and the highest risk was within 4 months of infection 1. In a worldwide meta-analysis of case-control studies, neonatal Bacille Calmette-Guérin (BCG) vaccination was shown to protect against TBM in children up to 5 years of age with a pooled efficacy of 73% 17,18. Non-specific symptoms and the accompanying difficulty with early diagnosis highlight the importance of TBM prevention with BCG vaccination in young children. TB preventative therapy following TB exposure is another key preventive strategy that substantially lowers the risk of developing TB disease in young children 19 but implementation is poor in high TB burden settings 20.
3. Epidemiology
The World Health Organization (WHO) estimates that 1.2 million children (<15 years of age) developed TB in 2019 21. Yet, only 523,000 of these were notified by TB programs globally that year, leaving more than half of all children with TB undiagnosed or diagnosed but not reported 21. Despite this large reporting gap, substantial progress has been made to strengthen paediatric TB surveillance since 2011, including reporting of age-disaggregated data on case notifications in 5-year age bands and also on outcomes for both drug-susceptible and drug-resistant paediatric TB since 2020 21. Despite current standard treatment and prevention strategies, the large number of children with undiagnosed TB (case detection gap) results in TB remains one of the top 10 causes of childhood mortality 22.
Childhood TB accounts for 5–20% of the total TB caseload in a population, depending on the population age structure, TB and HIV prevalence, and availability of preventative measures 23. Most low- and middle-income countries, typically those also affected most by TB, have a relatively young population (broad-based population pyramid) 24. In these countries, the annual risk of TB infection is high, with younger children having an increased risk of TB exposure and infection. As the risk for progression from TB infection to TB disease, and even more so to TBM, is age-related and disproportionately high in children younger than 2 years, more TB infections in this age group not only leads to more children who develop TB, but also to more children with TBM 1.
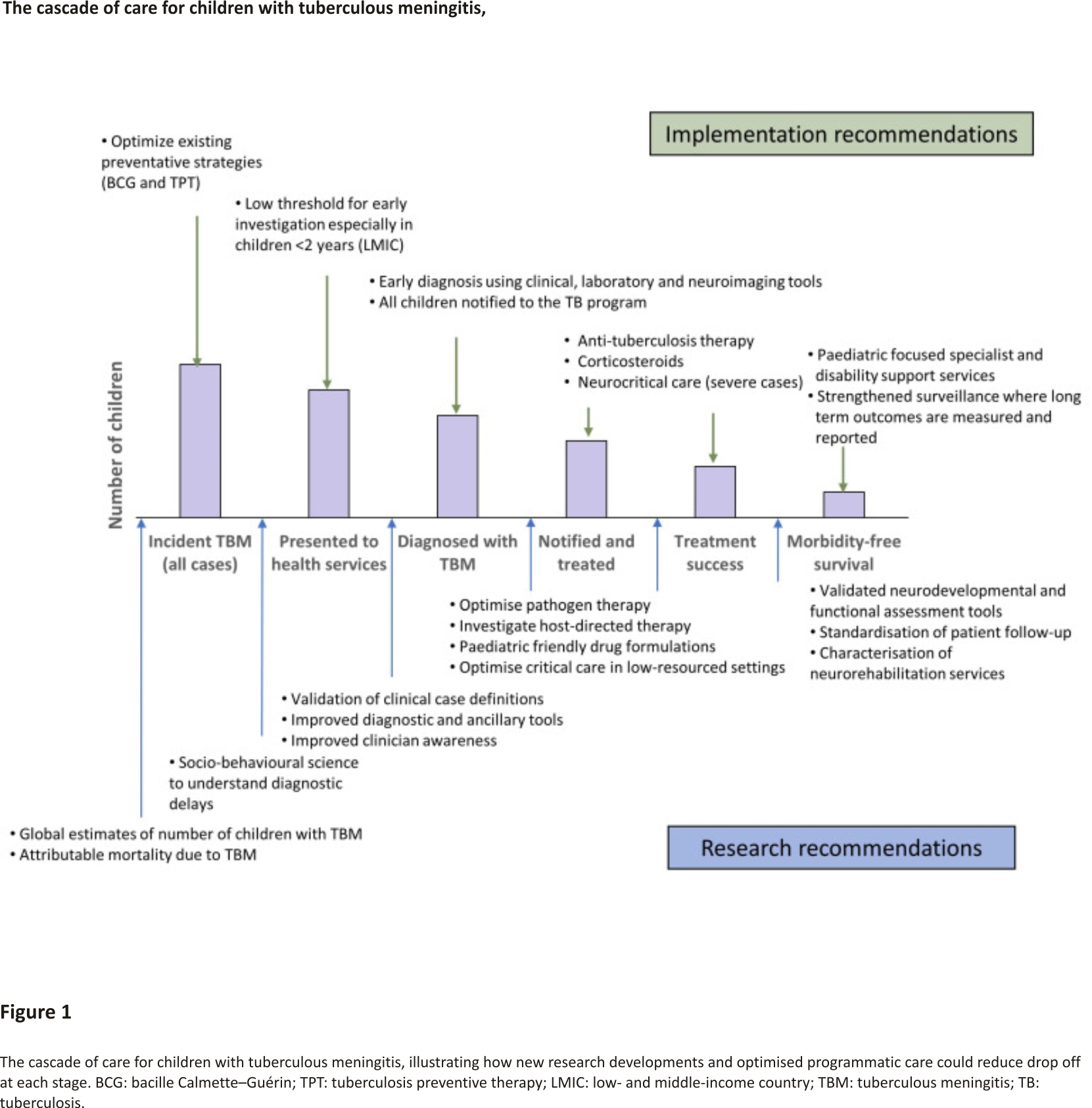
At present, there are no estimates for the number of children affected by TBM. Current TB surveillance data does not require reporting of severe forms of TB, such as TBM, and even the number of children diagnosed, treated and reported is not known. This limits our ability to raise an adequate and effective response to TBM in children 25. A study from Germany demonstrated that although the overall proportion of TB cases that were TBM was ~1%, this figure was 3.9% in children <5 years, 2.2% in children aged 5–9 years and 1.3% in children aged 10–14 years 26. If 2% of childhood TB is TBM, then 20,000 childhood TBM cases are to be estimated each year globally. Including surveillance of TBM as part of monitoring and evaluation of paediatric TB, care can help us measure and report both on the burden and outcomes of TBM in children and identify health system challenges. Given the substantial morbidity and often life-long disability suffered by children who survive, data on the burden of post-TBM health in children is critical to ensure adequate healthcare support is available to these children and their families. One way of better understanding the relationship between TBM in children and the health system response, is through cascade analysis. By evaluating drop-offs at each stage in the care cascade, programmatic challenges and research priorities can be identified (Figure 1). There is a sequential drop-off in numbers of TBM-infected children at each stage of care from presentation to health services to long-term outpatient monitoring. Combined with the real-world challenges at each of these stages, the true number of children with TBM who die or survive with sequelae is far greater than currently appreciated.
4. Diagnosis
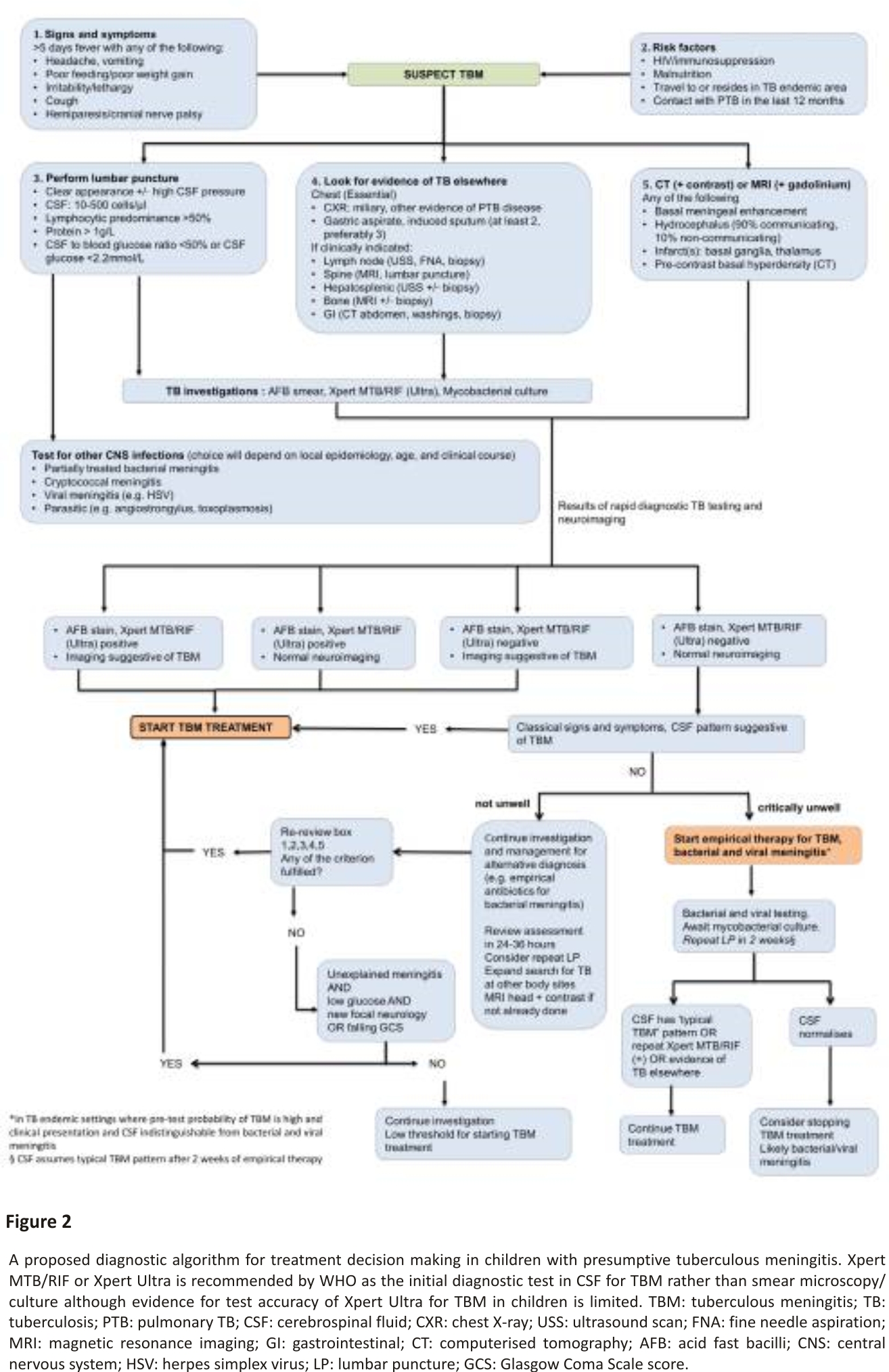
TBM in paediatric practice is diagnosed based on a combination of clinical, laboratory and neuroimaging findings (Figure 2) 27. Clinical prediction rules to distinguish TBM from other forms of meningitis have been proposed 28,29, whilst diagnostic algorithms such as the uniform research case definition 30 are designed for research purposes only. Clinical diagnostic rules are hampered by variable performance in different settings and a lack of external validation 31. To improve outcomes clinicians should maintain clinical suspicion and empirically treat suspected TBM without waiting for confirmatory results.
TBM can affect patients of all ages, however, the brunt of the disease is felt in early childhood with peak incidence commonly between 2 to 4 years 32 when the brain is still developing. It is difficult to make an early clinical diagnosis of TBM in childhood, subsequently resulting in delayed diagnosis and treatment, with an often inevitable poor outcome 3,32,33. The classical presentation of TBM is as a subacute meningitic illness, however, neck stiffness is often absent early in the course of the disease 34. In order to recognize early-stage TBM in children, clinicians in TB-endemic settings must be aware that TBM most commonly presents with non-specific symptoms of general ill-health. However, the persistence of symptoms allows differentiation from other common illnesses with similar presentation (e.g., influenza) 35. Household exposure to an adult source case with pulmonary TB within the previous year should heighten suspicion of TBM.
Both neuroimaging and CSF analysis is essential in the diagnostic assessment of paediatric TBM (Figure 2). Computed tomography (CT) is more readily available than magnetic resonance imaging (MRI) in resource-constrained settings 36. Classic CT findings include pre-and post-contrast basal meningeal exudates, hydrocephalus, infarcts and tuberculomas 37. MRI is superior in the early identification of TBM as gadolinium enhancement can detect small leptomeningeal tuberculomas, and diffusion-weighting can detect early infarction, both not reflected on CT 38,39. Leucocytosis with lymphocyte predominance, elevated protein and abnormally decreased CSF glucose is highly suggestive of TBM 32,40,41. Both an absolute CSF glucose value of <2.2 mmol/L and CSF protein >1 g/L differentiate TBM from viral or no meningitis in children with good specificity, albeit with poor sensitivity. CSF to serum glucose ratio, infrequently performed, is essential to provide additional information value 42.
Mycobacterial confirmation in children with presumed TBM is difficult due to low CSF volumes obtained and the paucibacillary nature of TBM. CSF microscopy is hampered by low sensitivity 43 however yields may be improved by centrifugation and longer examination time. Even though the sensitivity of CSF culture is higher than microscopy, it remains sub-optimal and the result rarely influences clinical management due to delays of up to 8 weeks 31. Nucleic-acid amplification testing offers the prospect of a rapid and specific result, however, few tests have undergone validation. Xpert MTB/ RIF (Cepheid, Sunnyvale, CA, USA) is a commercial, real-time PCR-based assay for the detection of M. tuberculosis in clinical specimens. In 2015 Xpert MTB/RIF has been recommended by the WHO as an essential diagnostic test if TBM is suspected, however, caution is advised for its use as a ‘rule out’ test 44,45. The second-generation Xpert MTB/RIF, XpertUltra, detects TBM with marginally higher sensitivity than Xpert and poor negative predictive value in adults, meaning it cannot be used to rule out TBM 46,47,48. In children, the poor positive predictive value may be more of an issue. The meaning of trace positive results on XpertUltra also is not understood. In spite of these limitations, Xpert MTB/Rif or Xpert Ultra is able to provide a result in under 1.5 h; a crucial advantage when initiating early anti-tuberculosis therapy if positive (Figure 2). We propose a diagnostic algorithm for presumptive TBM in children which incorporates clinical, CSF, neuroimaging features and rapid diagnostic results and provides guidance for possible clinical scenarios, including when diagnostic tools are negative or inconclusive for TBM and the clinician is left to make a clinical judgement (Figure 2).
Diagnostic tests for TBM are relatively expensive and inaccessible in resource-constrained areas, invasive and perform poorly in isolation 30,49. Recent technological advances have made it possible to screen for many biomarkers in a minute volume of CSF. A three-marker CSF biosignature comprising IL-13, VEGF and cathelicidin LL-37, diagnosed childhood TBM with a sensitivity of 52%, specificity of 95%, with positive and negative predictive values of 91% and 66% respectively 50. Validation of this three-marker CSF biosignature in a different cohort revealed positive and negative predictive values of 90% and 59.5% respectively 49. In a study investigating potentially useful host CSF biomarkers in childhood TBM, a combination of IFN-γ, MPO and VEGF showed good accuracy (AUC = 0.97, up to 91.3% sensitivity and up to 100% specificity) 49. Low CSF tryptophan concentration is associated with survival in TBM patients 51. The CSF metabolome in TBM is also characterized by amino acids (besides for tryptophan), organic acids, nucleotides and carbohydrates, all linked to altered neuro -energetics 52,53,54. CSF metabolomics studies of paediatric TBM 55,56,57 are advancing from a proof-of-concept, exploratory phase towards validation and standardization as biomarkers 54. Another host-immune response testing (e.g., CSF IFN-γ release assays) has only moderate diagnostic accuracy 58 while systematic reviews on CSF adenosine deaminase levels concluded that heterogeneity in methods and data limit applicability in clinical practise 31,59,60. It remains to be seen whether biomarker-based approaches can be transformed into easy-to-use point-of-care diagnostic tests 61, especially, in resource-limited settings, but it is likely that the future of TBM diagnosis will require a combination of pathogen testing and host-immune biomarkers.
5. Treatment
Currently, the WHO recommends treatment for paediatric TBM similar to that used for pulmonary TB—isoniazid, rifampicin, and pyrazinamide plus ethambutol—given at standard doses, albeit with treatment extension from 6 to 12 months. This recommendation was provided with low quality of evidence prior to the implementation of GRADE procedures in WHO guidelines process 62. However, to be effective, drugs must be present at therapeutic concentrations at the site of disease. This is critically important early in treatment, to prevent mortality and neurocognitive disabilities. The latter is particularly important for children whose developing brains render them susceptible to brain injury from TBM or its treatment. Several first-line anti-tuberculosis drugs (e.g., rifampicin and ethambutol) have poor penetration across the blood-brain and the blood-CSF barriers 63. Rifampicin remains an essential medication for TBM 64 but standard dosing yields CSF concentrations that are below the minimal inhibitory concentration (MIC) for M. tuberculosis in a large proportion of patients, including children 65,66,67. A recent model-based meta-analysis using emerging data from adult trials showed a strong positive correlation between rifampicin concentrations and survival, with doses of at least 30 mg/kg predicted to improve survival substantially compared to 10 mg/kg 68. A dose of 35 mg/kg daily is being tested in a definitive Phase 3 trial 69 but higher mg/kg doses than that will be needed to achieve adult-equivalent exposures in children 70. In children, a small trial suggested that a dose increase to 30 mg/kg improved neurocognitive function compared to standard dosing 71.
While higher rifampicin exposures were associated with improved outcomes in Indonesia for adults, in a large adult study in Vietnam, isoniazid exposures correlated more strongly with survival 72. While isoniazid penetrates freely into CSF, patients with a fast n-acetyltransferase 2 (NAT2) genotype may experience sub-therapeutic concentrations. Pyrazinamide passes easily into CSF, but its contribution to TBM treatment remains poorly defined 73. Recently approved as an alternative to the standard of care, the ‘Cape Town regimen’ takes into account the pharmacokinetics of anti-TB drugs, including information about their CNS distribution. This regimen, used for paediatric TBM in South Africa for over 20 years, includes high-dose rifampicin, high-dose isoniazid, ethionamide, and pyrazinamide for 6 months 74. Following a recent systematic review and meta-analysis (3 studies, 1006 participants) showing improved mortality in children who received a 6-month intensified regimen for TBM compared to the standard 12 months, WHO issued a rapid communication on TB management suggesting that the shortened regimen could be used as an alternative in children and adolescents 75. Two paediatric clinical trials SURE (ISRCTN40829906) and TBM-KIDS (NCT02958709) are currently underway and will measure mortality, functional status and neurocognitive outcomes in children receiving shortened intensified drug regimen (optimised dosing of rifampicin, with isoniazid, pyrazinamide, and levofloxacin) versus the standard WHO regimen.
Second-line drugs may be needed either for drug-resistant TBM or to replace drugs that display poor CNS penetration or cause toxicity. Fluoroquinolones achieve high CSF concentrations and are generally safe in children, though there have been some reports of intracranial hypertension and seizures associated with these agents 76,77. Linezolid is used for CNS infections involving gram positive bacteria, and early data from cohort studies suggest this drug is also good for TBM 78,79,80 though its toxicities make use beyond 8 weeks challenging 81. Delamanid or pretomanid may be useful in TBM 82 and bedaquiline appears to have similar free-drug concentrations in CSF as in plasma 83, but the place of these newer anti-TB drugs in TBM remains to be established. Another clinically challenging situation is HIV-associated TBM. While in adults, antiretroviral therapy (ART) is typically delayed until the intensive phase of TBM treatment is complete 84 the right timing for introduction of ART in children, who tend to have more rapid progression to severe HIV, is unknown.
To address the pathological impact of the host response, adjunctive steroids are given as the standard of care in TBM. Additional host-directed therapies may be useful for select patients, including aspirin or low-dose thalidomide 85,86,87. Whilst RCTs evaluating aspirin are underway, the role of non-corticosteroids adjuvant agents is not yet established. Management of CNS complications of TBM – infarcts, seizures, hydrocephalus – remains central to the care of individuals with this disease, and some published guidance documents have been produced to aid clinicians 88,89.
6. Outcomes
Despite the advent of anti-TB chemotherapy and corticosteroids most deaths (95%) from TBM will still occur by 6 months 69. An observational report in 1961 on long-term outcomes in children with TBM, was the first to highlight persistent neurodevelopmental sequelae years following completion of TB treatment 90. Younger age (<2 years of age) and more severe disease at diagnosis was associated with worse neurological sequelae 90. Whilst there has been recent progress in management of TBM, it has not yet translated into outcome benefits in clinical practice. Adjuvant corticosteroids reduce mortality in TBM, but they do not reduce neurodisability in survivors 91.
A concerning 65% of children who survive TBM do so with some form of disability ranging from motor, sensory, cognitive to developmental deficits 3,92. This is likely to be under-identified due to lack of surveillance and a standardised approach to follow-up. Reported outcomes vary depending on the prevalence of risk factors including HIV co-infection, drug resistance 93 severe hydrocephalus, cerebral infarct, brainstem dysfunction, raised intracranial pressure and malnutrition 32,33,94,95,96. Lack of access to supportive care and neurorehabilitation in low-resourced, TB-endemic settings further exacerbate poor outcomes 97. Whilst reasons for poor outcomes are likely to be multifactorial, diagnosis before the onset of coma remains the most crucial factor predicting survival and favourable outcomes 98.
Predicting prognosis in children with TBM is difficult owing to its insidious onset, diversity of immunopathology under various genetic influences and limited knowledge about the durability of brain injury in children. Emerging evidence, using biomarkers of cerebral injury usually seen in neurodegenerative disease, suggest that brain injury in TBM increases over time and lasts long after completion of TB treatment and corticosteroids 12. New molecular techniques (i.e., transcriptomics, proteomic and metabolomics) characterising signatures linking clinical phenotypes with TBM outcomes will likely advance knowledge in markers of prognostication 16,51. Currently, the best association with 6-month neurological outcomes is a clinical staging of severity using refined MRC grading system one week after diagnosis 99.
Long-term outcomes in children with TBM include cerebral palsy (e.g., hemiplegia) vision impairment (e.g., blindness), hearing loss, cognitive impairment (e.g., learning capacity), chronic seizure disorder, behavioural disturbance (e.g., Attention Deficit Hyperreactivity Disorder) and developmental disability 32,100. Notably, neurocognitive deficits can occur without accompanying physical disability 33. Neurocognitive and functional impairment is difficult to fully characterise due to diversity of phenotypes and recovery across age groups, loss to follow-up and a lack of testing specifically developed or validated for evaluation in TBM 101.
7. Conclusions
TBM in children remains a devastating disease, associated with substantial morbidity and mortality. It is challenging to diagnose and treat early; much of the damage has already occurred before the child is started on appropriate therapy. Our understanding of the pathophysiology and epidemiology of the condition is improving, and novel diagnostic approaches are being developed. New anti-TB drug regimens and dosing strategies are under evaluation, emerging host-directed therapies are being explored and supportive care is improving. However, substantial gains could be made by strengthening existing health systems, allowing earlier diagnosis and appropriate treatment and optimised surveillance
Author Contributions
Conceptualisation, J.H. and J.A.S.; writing—original draft preparation Y.-N.A., K.d.P., K.E.D., R.S., J.H. and J.A.S.; writing—review and editing J.H. and J.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgements
J.H. is supported by the Wellcome Trust, U.K. JAS is supported by a Clinician Scientist Fellowship jointly funded by the U.K. Medical Research Council (MRC) and the U.K. Department for International Development (DFID) under the MRC/DFID Concordat agreement (MR/R007942/1). KDP is supported by the Fogarty International Center of the National Institutes of Health under Award Number K43TW011006. KED is supported by NIAID/NIH Grant K24AI150349. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Hesseling, A.C.; Obihara, C.C.; Starke, J.J.; A Enarson, D.; Donald, P.R.; Beyers, N. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int. J. Tuberc. Lung Dis. 2004, 8, 392–402.
2. Lincoln, E.M. Tuberculous meningitis in children; with special reference to serous meningitis; tuberculous meningitis. Am. Rev. Tuberc. 1947, 56, 75–94.
3. Chiang, S.; Khan, F.; Milstein, M.; Tolman, A.W.; Benedetti, A.; Starke, J.R.; Becerra, M.C. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 947–957.
4. Davis, A.G.; Rohlwink, U.K.; Proust, A.; Figaji, A.A.; Wilkinson, R.J. The pathogenesis of tuberculous meningitis. J. Leukoc. Biol. 2019, 105, 267–280.
5. Jain, S.K.; Paul-Satyaseela, M.; Lamichhane, G.; Kim, K.S.; Bishai, W.R. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 2006, 193, 1287–1295.
6. Rich, A.R. The Pathogenesis of Tuberculosis; Charles C Thomas: Springfield, IL, USA, 1946.
7. Nguyen, L.; Pieters, J. The Trojan horse: Survival tactics of pathogenic mycobacteria in macrophages. Trends Cell. Biol. 2005, 15, 269–276.
8. Be, N.A.; Bishai, W.R.; Jain, S.K. Role of Mycobacterium tuberculosis pknD in the pathogenesis of central nervous system tuberculosis. BMC Microbiol. 2012, 12, 7.
9. Rich, A.R.; McCordick, H.A. The pathogenesis of tuberculous meningitis. Bull. Johns Hopkins Hosp. 1933, 52, 5–37.
10. Donald, P.R.; Schaaf, H.S.; Schoeman, J.F. Tuberculous meningitis and miliary tuberculosis: The Rich focus revisited. J. Infect. 2005, 50, 193–195.
11. Zaharie, S.D.; Franken, D.J.; van der Kuip, M.; van Elsland, S.; de Bakker, B.S.; Hagoort, J.; Roest, S.L.; van Dam, C.S.; Timmer, C.; Solo-mons, R.; et al. The immunological architecture of granulomatous inflammation in central nervous system tuberculosis. Tuberculosis 2020, 125, 102016.
12. Rohlwink, U.K.; Mauff, K.; Wilkinson, K.A.; Enslin, N.; Wegoye, E.; Wilkinson, R.; Figaji, A. Biomarkers of Cerebral Injury and Inflammation in Pediatric Tuberculous Meningitis. Clin. Infect. Dis. 2017, 65, 1298–1307.
13. Cresswell, F.V.; Davis, A.G.; Sharma, K.; Basu Roy, R.; Ganiem, A.R.; Kagimu, E.; Solomons, R.; Wilkinson, R.J.; Bahr, N.C.; Thuong, N.T.T. Recent Developments in Tuberculous Meningitis Pathogenesis and Diagnostics. Wellcome Open Res. 2019, 4, 164.
14. Manyelo, C.M.; Chegou, N.N.; Seddon, J.A.; Snyders, C.I.; Mutavhatsindi, H.; Manngo, P.M.; Walzl, G.; Stanley, K.; Solomons, R.S. Serum and cerebrospinal fluid host proteins indicate stroke in children with tuberculous meningitis. PLoS ONE 2021, 16, e0250944.
15. Thuong, N.T.T.; Heemskerk, D.; Tram, T.T.B.; Thao, L.T.P.; Ramakrishnan, L.; Ha, V.T.N.; Bang, N.D.; Chau, T.T.H.; Lan, N.H.; Caws, M.; et al. Leukotriene A4 Hydrolase Genotype and HIV Infection Influence Intracerebral Inflammation and Survival from Tuberculous Meningitis. J. Infect. Dis. 2017, 215, 1020–1028.
16. Rohlwink, U.K.; Figaji, A.; Wilkinson, K.A.; Horswell, S.; Sesay, A.K.; Deffur, A.; Enslin, N.; Solomons, R.; Van Toorn, R.; Eley, B.; et al. Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat. Commun. 2019, 10, 3767.
17. Trunz, B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180.
18. Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994, 271, 698–702.
19. Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R.; Pediatric TBCSC. The risk of tuberculosis in children after close exposure: A systematic review and individual-participant meta-analysis. Lancet 2020, 395, 973–984.
20. Szkwarko, D.; Hirsch-Moverman, Y.; Du Plessis, L.; Du Preez, K.; Carr, C.; Mandalakas, A.M. Child contact management in high tuberculosis burden countries: A mixed-methods systematic review. PLoS ONE 2017, 12, e0182185.
21. World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
22. Dodd, P.J.; Yuen, C.M.; Sismanidis, C.; Seddon, J.A.; Jenkins, H.E. The global burden of tuberculosis mortality in children: A mathematical modelling study. Lancet Glob. Health 2017, 5, e898–e906.
23. Seddon, J.A.; Jenkins, H.E.; Liu, L.; Cohen, T.; Black, R.E.; Vos, T.; Becerra, M.C.; Graham, S.M.; Sismanidis, C. Counting children with tuberculosis: Why numbers matter. Int. J. Tuberc. Lung Dis. 2015, 19 (Suppl. 1), 9–16.
24. Donald, P.R. Childhood tuberculosis: The hidden epidemic. Int. J. Tuberc. Lung Dis. 2004, 8, 627–629.
25. Basu Roy, R.; Bakeera-Kitaka, S.; Chabala, C.; Gibb, D.M.; Huynh, J.; Mujuru, H.; Sankhyan, N.; Seddon, J.A.; Sharma, S.; Singh, V.; et al. Defeating Paediatric Tuberculous Meningitis: Applying the WHO “Defeating Meningitis by 2030: Global Roadmap”. Microorganisms 2021, 9, 857.
26. Ducomble, T.; Tolksdorf, K.; Karagiannis, I.; Hauer, B.; Brodhun, B.; Haas, W.; Fiebig, L. The burden of extrapulmonary and meningitis tuberculosis: An investigation of national surveillance data, Germany, 2002 to 2009. Euro Surveill. 2013, 18, 20436.
27. Solomons, R.S.; Visser, D.H.; Marais, B.J.; Schoeman, J.F.; van Furth, A.M. Diagnostic accuracy of a uniform research case definition for TBM in children: A prospective study. Int. J. Tuberc. Lung Dis. 2016, 20, 903–908.
28. Goenka, A.; Jeena, P.M.; Mlisana, K.; Solomon, T.; Spicer, K.; Stephenson, R.; Verma, A.; Dhada, B.; Griffiths, M.J. Rapid Accurate Identification of Tuberculous Meningitis Among South African Children Using a Novel Clinical Decision Tool. Pediatr. Infect. Dis. J. 2018, 37, 229–234.
29. Thwaites, G.E.; Chau, T.T.; Stepniewska, K.; Phu, N.; Chuong, L.; Sinh, D.; White, N.; Parry, C.; Farrar, J. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 2002, 360, 1287–1292. [Google Scholar] [CrossRef]
30. Marais, S.; Thwaites, G.; Schoeman, J.F.; Torok, M.E.; Misra, U.K.; Prasad, K.; Donald, P.R.; Wilkinson, R.J.; Marais, B.J. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 2010, 10, 803–812.
31. Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; van Crevel, R.; Mai, N.T.H.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R.; et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017, 13, 581–598.
32. van Well, G.T.; Paes, B.F.; Terwee, C.B.; Springer, P.; Roord, J.J.; Donald, P.R.; van Furth, A.M.; Schoeman, J.F. Twenty years of pediatric tuberculous meningitis: A retrospective cohort study in the western cape of South Africa. Pediatrics 2009, 123, e1–e8.
33. Schoeman, J.; Wait, J.; Burger, M.; van Zyl, F.; Fertig, G.; van Rensburg, A.J.; Springer, P.; Donald, P. Long-term follow up of childhood tuberculous meningitis. Dev. Med. Child. Neurol. 2002, 44, 522–526.
34. Thwaites, G.E.; van Toorn, R.; Schoeman, J. Tuberculous meningitis: More questions, still too few answers. Lancet Neurol. 2013, 12, 999–1010.
35. Solomons, R.; Grantham, M.; Marais, B.J.; van Toorn, R. IMCI indicators of childhood TBM at primary health care level in the Western Cape Province of South Africa. Int. J. Tuberc. Lung Dis. 2016, 20, 1309–1313.
36. van Toorn, R.; Solomons, R. Update on the diagnosis and management of tuberculous meningitis in children. Semin. Pediatric Neurol. 2014, 21, 12–18.
37. Andronikou, S.; Smith, B.; Hatherhill, M.; Douis, H.; Wilmshurst, J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr. Radiol. 2004, 34, 876–885.
38. van der Merwe, D.J.; Andronikou, S.; Van Toorn, R.; Pienaar, M. Brainstem ischemic lesions on MRI in children with tuberculous meningitis: With diffusion-weighted confirmation. Childs Nerv. Syst. 2009, 25, 949–954.
39. Pienaar, M.; Andronikou, S.; van Toorn, R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv. Syst. 2009, 25, 941–947.
40. Thwaites, G.; Fisher, M.; Hemingway, C.; Scott, G.; Solomon, T.; Innes, J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J. Infect. 2009, 59, 167–187.
41. Torok, M.E.; Chau, T.T.; Mai, P.P.; Phong, N.D.; Dung, N.T.; Van Chuong, L.; Lee, S.J.; Caws, M.; De Jong, M.D.; Hien, T.T.; et al. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS ONE 2008, 3, e1772.
42. Solomons, R.S.; Visser, D.H.; Donald, P.R.; Marais, B.J.; Schoeman, J.F.; van Furth, A.M. The diagnostic value of cerebrospinal fluid chemistry results in childhood tuberculous meningitis. Childs Nerv. Syst. 2015, 31, 1335–1340.
43. Thwaites, G.; Chau, T.T.; Mai, N.T.; Drobniewski, F.; McAdam, K.; Farrar, J. Tuberculous meningitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 289–299.
44. Bahr, N.C.; Marais, S.; Caws, M.; van Crevel, R.; Wilkinson, R.J.; Tyagi, J.S.; Thwaites, G.E.; Boulware, D.R. GeneXpert MTB/Rif to Diagnose Tuberculous Meningitis: Perhaps the First Test but not the Last. Clin. Infect. Dis. 2016, 62, 1133–1135.
45. Boyles, T.H.; Thwaites, G.E. Appropriate use of the Xpert(R) MTB/RIF assay in suspected tuberculous meningitis. Int. J. Tuberc. Lung Dis. 2015, 19, 276–277.
46. Cresswell, F.V.; Tugume, L.; Bahr, N.C.; Kwizera, R.; Bangdiwala, A.S.; Musubire, A.K.; Rutakingirwa, M.K.; Kagimu, E.; Nuwagira, E.; Mpoza, E.; et al. Xpert MTB/RIF Ultra for the diagnosis of HIV-associated tuberculous meningitis: A prospective validation study. Lancet Infect. Dis. 2020, 20, 308–317.
47. Donovan, J.; Thu, D.D.A.; Phu, N.H.; Dung, V.T.M.; Quang, T.P.; Nghia, H.D.T.; Oanh, P.K.N.; Nhu, T.B.; Chau, N.V.V.; Ha, V.T.N.; et al. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for the diagnosis of tuberculous meningitis: A prospective, randomised, diagnostic accuracy study. Lancet Infect. Dis. 2020, 20, 299–307.
48. Donovan, J.; Cresswell, F.V.; Thuong, N.T.T.; Boulware, D.R.; E Thwaites, G.; Bahr, N.C.; E Aarnoutse, R.; Anderson, S.T.B.; Bang, N.D.; Boyles, T.; et al. Xpert MTB/RIF Ultra for the Diagnosis of Tuberculous Meningitis: A Small Step Forward. Clin. Infect. Dis. 2020, 71, 2002–2005.
49. Manyelo, C.M.; Solomons, R.S.; Snyders, C.I.; Manngo, P.M.; Mutavhatsindi, H.; Kriel, B.; Stanley, K.; Walzl, G.; Chegou, N.N. Application of Cerebrospinal Fluid Host Protein Biosignatures in the Diagnosis of Tuberculous Meningitis in Children from a High Burden Setting. Mediat. Inflamm. 2019, 2019, 7582948.
50. Visser, D.H.; Solomons, R.S.; Ronacher, K.; van Well, G.T.; Heymans, M.W.; Walzl, G.; Chegou, N.N.; Schoeman, J.F.; van Furth, A.M. Host immune response to tuberculous meningitis. Clin. Infect. Dis. 2015, 60, 177–187.
51. van Laarhoven, A.; Dian, S.; Aguirre-Gamboa, R.; Avila-Pacheco, J.; Ricano-Ponce, I.; Ruesen, C.; Annisa, J.; Koeken, V.A.C.M.; Chaidir, L.; Li, Y.; et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: An observational cohort study. Lancet Infect. Dis. 2018, 18, 526–535.
52. Isaiah, S.; Loots, D.T.; Solomons, R.; van der Kuip, M.; Tutu Van Furth, A.M.; Mason, S. Overview of Brain-to-Gut Axis Exposed to Chronic CNS Bacterial Infection(s) and a Predictive Urinary Metabolic Profile of a Brain Infected by Mycobacterium tuberculosis. Front. Neurosci. 2020, 14, 296.
53. Mason, S. Lactate Shuttles in Neuroenergetics- Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11, 43.
54. Mason, S.; Solomons, R. CSF Metabolomics of Tuberculous Meningitis: A Review. Metabolites 2021, 11, 661.
55. Mason, S.; van Furth, A.M.; Mienie, L.J.; Engelke, U.F.H.; Wevers, R.A.; Solomons, R.; Reinecke, C.J. A hypothetical astrocyte-microglia lactate shuttle derived from a (1)H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics 2015, 11, 822–837.
56. Mason, S.; Reinecke, C.J.; Solomons, R. Cerebrospinal Fluid Amino Acid Profiling of Pediatric Cases with Tuberculous Meningitis. Front. Neurosci. 2017, 11, 534.
57. van Zyl, C.W.; Loots, D.T.; Solomons, R.; van Reenen, M.; Mason, S. Metabolic characterization of tuberculous meningitis in a South African paediatric population using (1)H NMR metabolomics. J. Infect. 2020, 81, 743–752.
58. Yu, J.; Wang, Z.J.; Chen, L.H.; Li, H.H. Diagnostic accuracy of interferon-gamma release assays for tuberculous meningitis: A meta-analysis. Int. J. Tuberc. Lung Dis. 2016, 20, 494–499.
59. Tuon, F.F.; Higashino, H.R.; Lopes, M.I.; Litvoc, M.N.; Atomiya, A.N.; Antonangelo, L.; Leite, O.M. Adenosine deaminase and tuberculous meningitis-a systematic review with meta-analysis. Scand J. Infect. Dis 2010, 42, 198–207.
60. Xu, H.B.; Jiang, R.H.; Li, L.; Sha, W.; Xiao, H.P. Diagnostic value of adenosine deaminase in cerebrospinal fluid for tuberculous meningitis: A meta-analysis. Int. J. Tuberc. Lung Dis. 2010, 14, 1382–1387.
61. Manyelo, C.M.; Solomons, R.S.; Walzl, G.; Chegou, N.N. Tuberculous Meningitis: Pathogenesis, Immune Responses, Diagnostic Challenges, and the Potential of Biomarker-Based Approaches. J. Clin. Microbiol. 2021, 59, 3.
62. World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children, 2nd ed.; WHO: Geneva, Switzerland, 2014; Available online: http://apps.who.int/ iris/bitstream/handle/10665/112360/9789241548748_eng.pdf
63. Donald, P.R. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis 2010, 90, 279–292.
64. Vinnard, C.; King, L.; Munsiff, S.; Crossa, A.; Iwata, K.; Pasipanodya, J.; Proops, D.; Ahuja, S. Long-term Mortality of Patients with Tuberculous Meningitis in New York City: A Cohort Study. Infect. Dis. Soc. Am. 2017, 64, 401–407.
65. Cresswell, F.V.; Meya, D.B.; Kagimu, E.; Grint, D.; Te Brake, L.; Kasibante, J.; Martyn, E.; Rutakingirwa, M.; Quinn, C.M.; Okirwoth, M.; et al. High-Dose Oral and Intravenous Rifampicin for the Treatment of Tuberculous Meningitis in Predominantly Human Immunodeficiency Virus (HIV)-Positive Ugandan Adults: A Phase II Open-Label Randomized Controlled Trial. Clin. Infect. Dis. 2021, 73, 876–884.
66. Pouplin, T.; Bang, N.D.; Toi, P.V.; Phuong, P.N.; Dung, N.H.; Duong, T.N.; Caws, M.; Thwaites, G.E.; Tarning, J.; Day, J.N. Naive-pooled pharmacokinetic analysis of pyrazinamide, isoniazid and rifampicin in plasma and cerebrospinal fluid of Vietnamese children with tuberculous meningitis. BMC Infect. Dis. 2016, 16, 144.
67. Ruslami, R.; Gafar, F.; Yunivita, V.; Parwati, I.; Ganiem, A.R.; Aarnoutse, R.E.; Wilffert, B.; Alffenaar, J.W.C. Pharmacokinetics and safety/tolerability of isoniazid, rifampicin and pyrazinamide in children and adolescents treated for tuberculous meningitis. Arch. Dis. Child. 2022, 107, 70–77.
68. Svensson, E.M.; Dian, S.; Te Brake, L.; Ganiem, A.R.; Yunivita, V.; van Laarhoven, A.; Van Crevel, R.; Ruslami, R.; Aarnoutse, R.E. Model-Based Meta-analysis of Rifampicin Exposure and Mortality in Indonesian Tuberculous Meningitis Trials. Clin. Infect. Dis. 2020, 71, 1817–1823.
69. Marais, S.; Cresswell, F.V.; Hamers, R.L.; Te Brake, L.H.M.; Ganiem, A.R.; Imran, D.; Bangdiwala, A.; Martyn, E.; Kasibante, J.; Kagimu, E.; et al. High dose oral rifampicin to improve survival from adult tuberculous meningitis: A randomised placebo-controlled double-blinded phase III trial (the HARVEST study). Wellcome Open Res. 2019, 4, 190.
70. Garcia-Prats, A.J.; Svensson, E.M.; Winckler, J.; Draper, H.R.; Fairlie, L.; van der Laan, L.E.; Masenya, M.; Schaaf, H.S.; Wiesner, L.; Norman, J.; et al. Pharmacokinetics and safety of high-dose rifampicin in children with TB: The Opti-Rif trial. J. Antimicrob. Chemother. 2021, 76, 3237–3246.
71. Valvi, C. OA24–763–21 High-dose rifampicin with or without levofloxacin for the treatment of paediatric tuberculous meningitis. In Proceedings of the 52nd Union World Conference on Lung Health, Virtual Event, 21–23 October 2021.
72. Ding, J.; Thuy Thuong Thuong, N.; Pham, T.V.; Heemskerk, D.; Pouplin, T.; Tran, C.T.H.; Nguyen, M.T.H.; Nguyen, P.H.; Phan, L.P.; Nguyen, C.V.V.; et al. Pharmacokinetics and Pharmacodynamics of Intensive Antituberculosis Treatment of Tuberculous Meningitis. Clin. Pharmacol. Ther. 2020, 107, 1023–1033.
73. Torok, M.E.; Aljayyoussi, G.; Waterhouse, D.; Chau, T.T.H.; Mai, N.T.H.; Phu, N.H.; Hien, T.T.; Hope, W.; Farrar, J.J.; Ward, S.A. Suboptimal Exposure to Anti-TB Drugs in a TBM/HIV+ Population Is Not Related to Antiretroviral Therapy. Clin. Pharmacol. Ther. 2018, 103, 449–457.
74. van Toorn, R.; Schaaf, H.S.; Laubscher, J.A.; van Elsland, S.L.; Donald, P.R.; Schoeman, J.F. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatric Infect. Dis. J. 2014, 33, 248–252.
75. World Health Organization. Rapid Communication on Updated Guidance on the Management of Tuberculosis in Children and Adolescents. Available online: https://www.who.int/ publications /i/item/9789240033450
76. Kalita, J.; Bhoi, S.K.; Betai, S.; Misra, U.K. Safety and efficacy of additional levofloxacin in tuberculous meningitis: A randomized controlled pilot study. Tuberculosis 2016, 98, 1–6.
77. van der Laan, L.E.; Schaaf, H.S.; Solomons, R.; Willemse, M.; Mohamed, N.; Baboolal, S.O.; Hesseling, A.C.; van Toorn, R.; Garcia-Prats, A.J. Probable Levofloxacin-associated Secondary Intracranial Hypertension in a Child with Multidrug-resistant Tuberculosis. Pediatr. Infect. Dis. J. 2016, 35, 706–708.
78. Fang, M.T.; Su, Y.F.; An, H.R.; Zhang, P.Z.; Deng, G.F.; Liu, H.M.; Mao, Z.; Zeng, J.F.; Li, G.; Yang, Q.T.; et al. Decreased mortality seen in rifampicin/multidrug-resistant tuberculous meningitis treated with linezolid in Shenzhen, China. BMC Infect. Dis. 2021, 21, 1015.
79. Li, H.; Lu, J.; Liu, J.; Zhao, Y.; Ni, X.; Zhao, S. Linezolid is Associated with Improved Early Outcomes of Childhood Tuberculous Meningitis. Pediatric Infect. Dis. J. 2016, 35, 607–610.
80. Sun, F.; Ruan, Q.; Wang, J.; Chen, S.; Jin, J.; Shao, L.; Zhang, Y.; Zhang, W. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob. Agents Chemother. 2014, 58, 6297–6301.
81. Garcia-Prats, A.J.; Schaaf, H.S.; Draper, H.R.; Garcia-Cremades, M.; Winckler, J.; Wiesner, L.; Hesseling, A.C.; Savic, R.M. Pharmacokinetics, optimal dosing, and safety of linezolid in children with multidrug-resistant tuberculosis: Combined data from two prospective observational studies. PLoS Med. 2019, 16, e1002789.
82. Howell, P.J.B.; Upton, C.; Mvuna, N.; van Niekerk, C.; Everitt, D.; Olugbosi, M.; Conradi, F. Sterile tuberculous granuloma in a patient with XDR-TB treated with bedaquiline, pretomanid and linezolid. In Proceedings of the American Thoracic Society International Conference, Dallas, TX, USA, 17–22 May 2019.
83. Upton, C. TBS-02–01 Bedaquiline in cerebrospinal fluid. In Proceedings of the 52nd Union World Conference TB Science, Online, 19 October 2021.
84. Torok, M.E.; Yen, N.T.; Chau, T.T.; Mai, N.T.H.; Phu, N.H.; Mai, P.P.; Dung, N.T.; Chau, N.V.V.; Bang, N.D.; Tien, N.A.; et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin. Infect. Dis. 2011, 52, 1374–1383.
85. Schoeman, J.F.; Janse van Rensburg, A.; Laubscher, J.A.; Springer, P. The role of aspirin in childhood tuberculous meningitis. J. Child Neurol. 2011, 26, 956–962.
86. Schoeman, J.F.; Springer, P.; van Rensburg, A.J.; Swanevelder, S.; Hanekom, W.A.; Haslett, P.A.; Kaplan, G. Adjunctive thalidomide therapy for childhood tuberculous meningitis: Results of a randomized study. J. Child Neurol. 2004, 19, 250–257.
87. van Toorn, R.; Solomons, R.S.; Seddon, J.A.; Schoeman, J.F. Thalidomide Use for Complicated Central Nervous System Tuberculosis in Children: Insights from an Observational Cohort. Clin. Infect. Dis. 2021, 72, e136–e145.
88. Figaji, A.A.; Fieggen, A.G. The neurosurgical and acute care management of tuberculous meningitis: Evidence and current practice. Tuberculosis 2010, 90, 393–400.
89. Donovan, J.; Rohlwink, U.K.; Tucker, E.W.; Hiep, N.T.T.; Thwaites, G.E.; Figaji, A.A. Checklists to guide the supportive and critical care of tuberculous meningitis. Wellcome Open Res. 2019, 4, 163.
90. Lorber, J. Long-term follow-up of 100 children who recovered from tuberculous meningitis. Pediatrics 1961, 28, 778–791.
91. Prasad, K.; Singh, M.B.; Ryan, H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst. Rev. 2016, 4, CD002244.
92. Aulakh, R.; Chopra, S. Pediatric Tubercular Meningitis: A Review. J. Pediatr. Neurosci. 2018, 13, 373–382.
93. Daniel, B.D.; Grace, G.A.; Natrajan, M. Tuberculous meningitis in children: Clinical management & outcome. Indian J. Med. Res. 2019, 150, 117–130.
94. Nataprawira, H.M.; Ruslianti, V.; Solek, P.; Hawani, D.; Milanti, M.; Anggraeni, R.; Memed, F.S.; Kartika, A. Outcome of tuberculous meningitis in children: The first comprehensive retrospective cohort study in Indonesia. Int. J. Tuberc. Lung Dis. 2016, 20, 909–914.
95. Springer, P.; Swanevelder, S.; van Toorn, R.; van Rensburg, A.J.; Schoeman, J. Cerebral infarction and neurodevelopmental outcome in childhood tuberculous meningitis. Eur. J. Paediatr. Neurol. 2009, 13, 343–349.
96. Seddon, J.A.; Visser, D.H.; Bartens, M.; Jordaan, A.M.; Victor, T.C.; van Furth, A.M.; Schoeman, J.F.; Schaaf, H.S. Impact of drug resistance on clinical outcome in children with tuberculous meningitis. Pediatr. Infect. Dis. J. 2012, 31, 711–716.
97. Tucker, E.W.; Marais, S.; Seddon, J.A.; van Crevel, R.; Ganiem, A.R.; Ruslami, R.; Zhang, W.; Sun, F.; Zhou, X.; Solomons, R.S.; et al. International Survey Reveals Opportunities to Improve Tuberculous Meningitis Management and the Need for Standardized Guidelines. Open Forum Infect. Dis. 2020, 7, ofaa445.
98. Wang, M.S.; Zhao, M.; Liu, X.J. Risk factors for poor outcome in childhood tuberculous meningitis. Sci. Rep. 2021, 11, 8654.
100. van Toorn, R.; Springer, P.; Laubscher, J.A.; Schoeman, J.F. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int. J. Tuberc. Lung Dis. 2012, 16, 628–632.
99. Wait, J.W.; Schoeman, J.F. Behaviour profiles after tuberculous meningitis. J. Trop. Pediatr. 2010, 56, 166–171.
100. Davis, A.G.; Nightingale, S.; Springer, P.E.; Solomons, R.; Arenivas, A.; Wilkinson, R.J.; Anderson, S.T.; Chow, F.C. Neurocognitive and functional impairment in adult and paediatric tuberculous meningitis. Wellcome Open Res. 2019, 4, 178. [
Credits: Huynh J, Abo Y-N, du Preez K, Solomons R, Dooley KE, Seddon JA. Tuberculous Meningitis in Children: Reducing the Burden of Death and Disability. Pathogens. 2022; 11(1):38. https://doi.org/ 10.3390/pathogens11010038











