Minghao Li 1, Sheng-Li Xue 2,3, Xiaowen Tang 2,3, Jiayu Xu 4, Suning Chen 2,3, Yue Han 2,3, Huiying Qiu 2,3, Miao Miao 2,3, Nan Xu 1, Jingwen Tan 1, Liqing Kang 5, Zhou Yu 5, Xiaoyan Lou 5, Yang Xu 2,3, Jia Chen 2,3, Zhiqiang Yan 1, Weixing Feng 4, Depei Wu 6,7, Lei Yu 8
Abstract
The tumour burden (TB) is significantly related to the severity of cytokine release syndrome (CRS) caused by CAR-T cells, but its correlation with therapeutic efficacy has not been systematically studied. This study focused on the effects of the TB level on both the safety and efficacy of ssCART-19 as a treatment for r/r B-ALL. Taking the 5% tumour burden as the boundary, the study participants were divided into 2 groups, high and low tumour burden groups. Under this grouping strategy, the impacts of differential r/r B-ALL TBs on the clinical therapeutic efficacy (CR rate and long-term survival) and safety profiles after ssCART-19 cell treatment were analysed. 78 patients were reported in this study. The differential B-ALL TBs significantly affected the complete remission (CR) rates of patients treated with ssCART-19, with rates of 93.94% and 75.56% in the low and high TB groups, respectively (P = 0.0358). The effects of TBs on long-term therapeutic efficacy were further studied based on event-free survival (EFS) and overall survival (OS) profiles; both the OS and EFS of the low TB group were better than those of the high TB group, but the differences were not statistically significant. Importantly, the time points of TB measurement did not significantly affect the OS and EFS profiles regardless of whether the TBs were measured before or after fludarabine-cyclophosphamide (FC) preconditional chemotherapy. On the other hand, the severity of CRS was significantly correlated with the TB level (P = 0.0080), and the incidence of sCRS was significantly related to the TB level (the sCRS incidence increased as the TB level increased, P = 0.0224). Unexpectedly, the ssCART-19 cell expansion peaks were not significantly different (P = 0.2951) between the study groups. Patients with a low r/r B-ALL TB yield more net benefits from CAR-T treatment than those with a high TB in terms of safety and CR rate. These findings are critical and valuable for determining the optimal CAR-T cell treatment window for r/r B-ALL patients and will further the development of comprehensive and reasonable CAR-T cell treatment plans for r/r B-ALL patients with differential Tbs.
Introduction
As new immunotherapy, chimeric antigen receptor T (CAR-T) cell therapy has shown remarkable effects in refractory or relapsed B-cell acute lymphoblastic leukaemia (r/r B-ALL). Studies have shown that the complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate of CAR-T cell therapy can be as high as 90%1,2. However, despite the continuous development of CAR-T cell therapy, some insufficiencies remain regarding its long-term effectiveness and safety. For example, recurrence can occur in patients (more than 50%) treated with CAR-T cells within 12 months after the achievement of CR3. Moreover, serious side effects, such as cytokine release syndrome (CRS) and neurotoxicity, often occur during CAR-T cell therapy and can even cause patient death4,5,6,7,8. These problems have largely limited the clinical application of CAR-T cell therapy in r/r B-ALL. Therefore, identifying predictive and early indicators of the clinical efficacy, prognosis, and safety of CAR-T cells is of great significance for formulating a reasonable clinical CAR-T cell treatment plan.
Multiple studies have shown that numerous factors are potentially related to the efficacy of CAR-T cells9,10,11,12,13,14. For instance, Pan J reported that the threshold dose for CAR-T cell infusion was related to cell expansion and therapeutic efficacy15, and higher remission rates (55–94.3%) were shown to be achieved with doses exceeding 1 × 106/kg3. In addition, Fraietta, J. A found higher expression levels of memory-related genes in CAR-T cells from responsive chronic lymphocytic leukaemia (CLL) patients, while the genes with increased expression in CAR-T cells from nonresponsive patients were shown to be involved in effector cell differentiation, glycolysis, depletion, and apoptosis15. Qin, J. S also found that ibrutinib could transform CAR-T cells into memory cells, which thereby increased the clearance rate of CD19+ tumours in mice and prolonged the survival times of tumour-bearing mice16. Although some studies have reported that tumour burden factors are correlated with CR after CAR-T cell therapy and with the survival rate, their inconsistent patient treatment methods and CAR-T cell infusion doses potentially affect the accuracy of statistical analyses17. Therefore, standardizing and systematically studying the tumour burden impacts on the CAR-T cell treatment response and long-term efficacy are necessary and important.
Severe CRS (sCRS) and CAR-T cell-related neurotoxicity are common side effects of CAR-T cell treatment. Many factors have been reported to be associated with these side effects, including the dose used for CAR-T cell reinfusion, the costimulatory domain molecules on CAR-T cells, and the types and amounts of pro-inflammatory factors released8,11,12,17,18,19. Studies have also shown that the tumour burden is significantly related to the safety of CAR-T cell clinical treatment6,20. Davila, M. L analysed the correlations among 39 cytokines, the tumour burden prior to treatment and sCRS and found that the increased levels of 7 cytokines (interferon (IFN)-γ, interleukin (IL)-5, IL-6, IL-10, FIt-3L, granulocyte macrophage-colony stimulating factor (GM-CSF) and fractalkine) were related to the pretreatment tumour burden and to sCRS. Further analysis showed that the severity of CRS was significantly correlated with the tumour burden prior to 19-28z CAR-T cell infusion19. Similarly, Brentjens, R. J reported that the cytokine levels were correlated with the B-ALL tumour burden before treatment21. Moreover, Teachey, D. T found high levels of 24 cytokines, including IFNγ, IL6, sgp130, and sIL6R, within the first month after CAR-T cell infusion, which implied an intricate and high correlation with CRS7.
Based on the duality of the efficacy and side effects of CAR-T cell treatment, the ultimate benefit of CAR-T cell treatment depends on the net benefit yielded from balancing treatment efficacy and side effects. The tumour burden affects the safety of CAR-T cell clinical treatment, but its impact on CAR-T cell efficacy has not been investigated in a systematic and standardized manner. Therefore, we analysed the therapeutic outcomes of patients with r/r B-ALL treated with ssCART-19 cells, researching the correlations of the tumour burden with the CR rate, long-term survival (overall survival (OS) and event-free survival (EFS)), and clinical risk. This study aimed to elucidate reliable indicators that are predictive of clinical treatment efficacy and risks and to guide the formulation of a reasonable overall clinical treatment plan for patients with varying tumour burdens to maximize the benefit of CAR-T cell treatment.
Patient and methods
Study design and patient enrolment
This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University and conducted to assess the safety and efficacy of ssCART-19 cells (i.e., T cells expressing a CAR composed of an anti-CD19 single-chain antibody fragment with IL-6-specific shRNA) in patients with relapsed or refractory CD19+ B-cell malignancies. The primary endpoint was the safety of treating relapsed or refractory CD19+ B-cell malignancy patients with ssCART-19 cells, and adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.3. The secondary endpoint was the objective response rate at the end of the study. All the patients provided written informed consent, and the enrolled patients met the following inclusion criteria: diagnosed with CD19+ r/r B-ALL with sufficient organ function (left ventricular ejection fraction ≥0.5 as determined by echocardiography, creatinine < 1.6mg/dL, aspartate aminotransferase/aspartate aminotransferase < 3 × the upper limit of normal, bilirubin < 2.0 mg/dL) and a Karnofsky performance status ≥60 or an ECOG performance status ≤2. The study was registered at ClinicalTrials. gov (NCT03919240).
All patients underwent bone marrow evaluations before or after interim therapy and immediately before the T cell infusion.
Protocol treatment and assessments
After leukapheresis and ssCART-19 cell manufacturing, patients received conditioning chemotherapy consisting of fludarabine (30 mg per square metre of body surface area per day) and cyclophosphamide (Cy; 500 mg per square metre per day) on days − 5, − 4, and − 3 (FC treatment) before the administration of ssCART-19 cells at a dose of 5 × 106 cells per kilogram of body weight with split doses of 10%, 30%, and 60%. The patient’s body temperature, blood pressure, heart rate and pulse oxygen were monitored, and blood was drawn according to the clinical trial process to monitor the patient’s cytokine and CAR-T cell expansion levels in the peripheral blood. Blood test parameters, blood biochemistry parameters, ferritin, and C-reactive protein were also routinely monitored according to the protocol.
Response and toxicity assessment
CR was defined as less than 5% blasts in the bone marrow without myelosuppression, no circulating blasts in peripheral blood, and the absence of extramedullary disease, regardless of cell count recovery. A negative MRD status was defined as less than 0.01% bone marrow blasts assessed by multiparameter flow cytometry, and absence of genetic aberrants assessed by karyotype analysis or molecular detection. The relapsed disease was defined as loss of CR status after the achievement of CR, including haematological relapse (bone marrow ALL blasts > 5%) or extramedullary relapse (central nervous system, other sites)17. The analysis of overall survival used death as the event, and the analysis of event-free survival used the earliest of no response, relapse, or death as the event. Patients who did not have an event had their data censored for the analyses at the date at which they were last known to be alive.
CRS was graded according to the National Cancer Institute CTCAE version 4.03 and was considered to be severe at grades higher than or equal to 3.
CAR-T cell-related neurotoxicity was assessed according to the National Cancer Institute CTCAE version 4.03, and severe neurotoxic effects were defined as a seizure of any grade or a toxic effect of grade 3 or higher.
Manufacture of ssCART-19 cell products
The ssCART-19 cell manufacturing procedures were described previously22. Briefly, peripheral blood mononuclear cells were purified from the patient’s blood by gradient centrifugation using Lymphoprep (Oriental Hua Hui, Beijing, China), and their CD3+ T cells were then enriched by positive selection using magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany). The T cells were transduced with lentiviral supernatant, followed by activation in vitro using anti-CD3/CD28 monoclonal antibodies (Miltenyi Biotec) at 5% CO2 and 37 °C for 24 h. After transduction for 48 h, the CAR-T cells were cultured and expanded in an AIM-V T cell medium (Gibco, Grand Island, NY, USA), which contained 100 IU/ml recombinant human IL-2 (PeproTech, Rocky Hill, NJ, USA), 5 ng/ml recombinant human IL-7 (PeproTech), 5 ng/ml recombinant human IL-15 (PeproTech) and 10% autologous plasma, at 5% CO2 and 37 °C for 14 days.
Cytokine concentration assessment
Cytokines were measured using the Th1/Th2 Cytometric Bead Array Kit II (BD Bioscience) according to the manufacturer’s instructions. In brief, serum was collected from patients at different time points. The cytokine-capture microspheres were first mixed and then incubated with serum samples and fluorophore-labelled antibodies for 3 h before being washed and evaluated by flow cytometry (Thermo Fisher). The concentration of each cytokine was calculated from standard curves.
Quantification of ssCART-19 cell expansion and maintenance
The expansion and persistence of the ssCART-19 cells were monitored by real-time quantitative PCR (qPCR). All patients’ peripheral blood samples have obtained at different time points. The corresponding DNA has amplified with primers and probes complementary to specific sequences within the lentiviral vector followed by extracted from the peripheral blood samples. qPCR has been performed using the ABI 7500 Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, USA), and calculation of the CAR-T copy number depends on the standard curve established with the plasmid encoding the transgene23.
Flow cytometry assays
CAR-T cell differentiation stages were assessed by flow cytometry. CAR-T cells were harvested and washed twice with 1 ml of phosphate-buffered saline containing 2% foetal bovine serum (FBS; Gibco), followed by incubation with the following antibodies: APC-Cy7-conjugated anti-CD8 (344714, BioLegend, California, USA), Alexa Fluor 700-conjugated anti-CD4 (56004942, eBioscience), PE-conjugated anti-CCR7 (353204, BioLegend), PerCP-Cy5.5-conjugated anti-CD45RA (304122, BioLegend), and PE-Cy7-conjugated anti-CD127 (25-1287-42, eBioscience)24.
Statistical analyses
The EFS and OS data in this study were analysed using the Kaplan–Meier log-rank test. T-tests (paired or unpaired), the chi-square test and Fisher’s exact test were also used in this study. P values less than 0.05 indicated statistical significance.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics review committee of the First Affiliated Hospital of Soochow University. All participants provided written informed consent.
Results
Patient characteristics
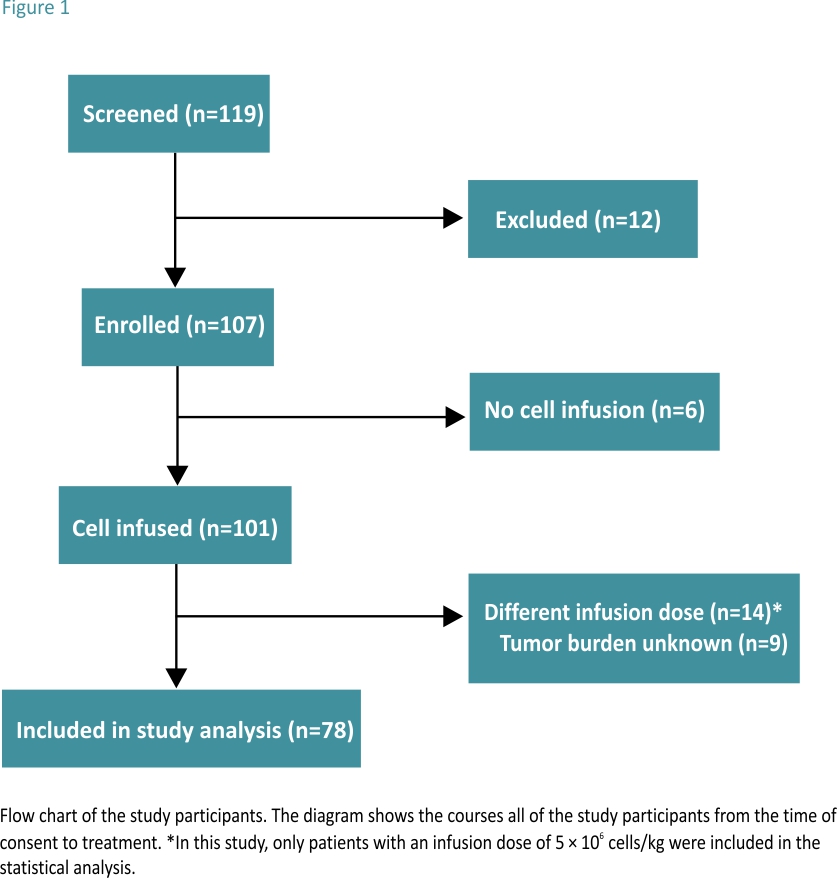
A total of 107 patients with r/r B-ALL were enrolled between February 2017 and May 2020. Among them, 78 patients were treated with ssCART-19 cells and were included in the statistical analysis, while the remaining 29 patients were not included in the analysis. The specific exclusion criteria are shown in Fig. 1.
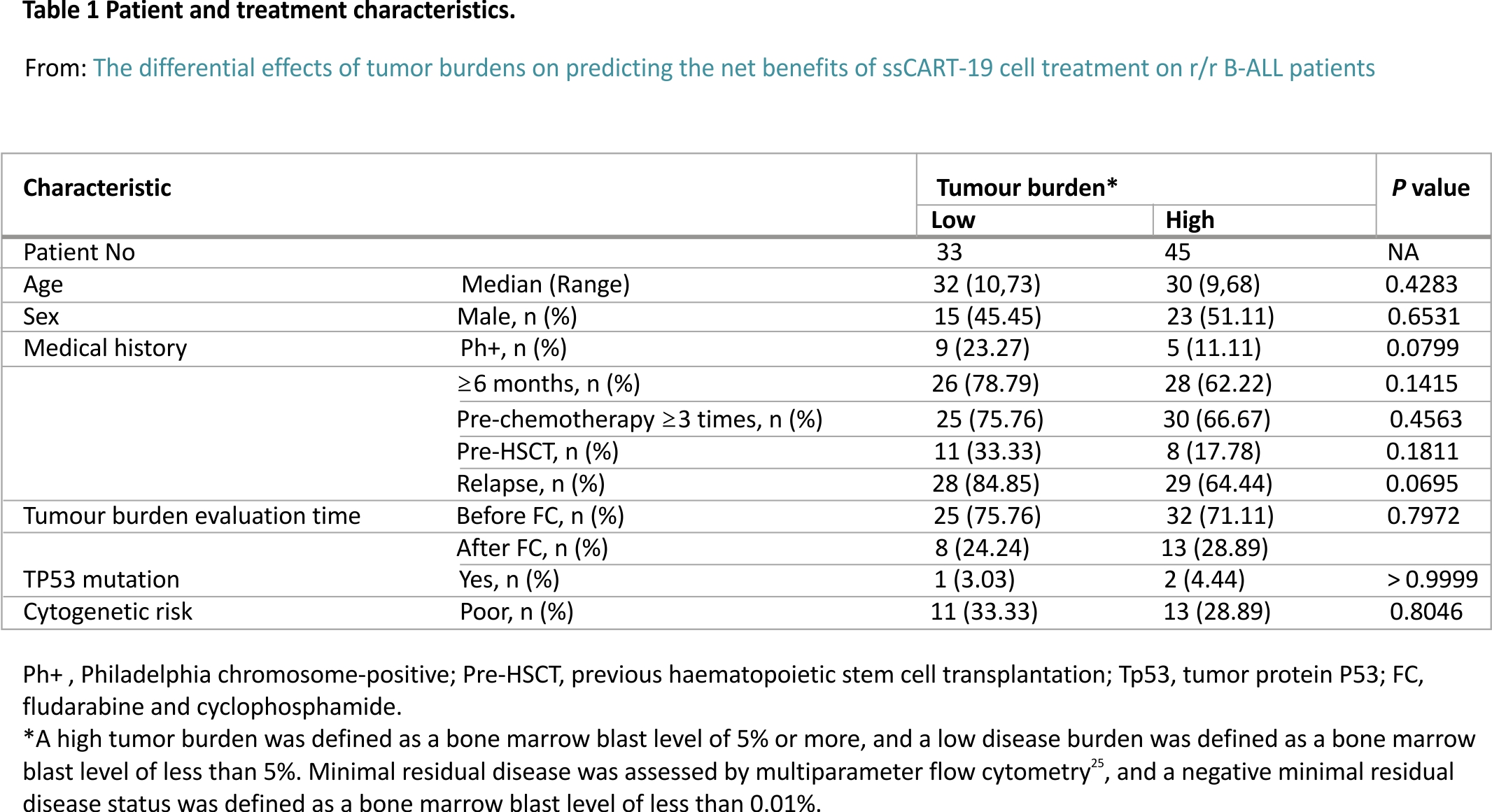
All 78 patients were treated with FC. According to the patient’s tumour burden before CAR-T cell reinfusion, the 78 patients were divided into two groups. Thirty-three patients were included in the low tumour burden group and had the minimal residual disease (MRD) with bone marrow blast percentages ranging from 0.01 to less than 5; the remaining 45 patients were in the high tumour burden group, with bone marrow blasts percentages equalling or exceeding 5.
Among the 33 patients in the low tumour burden group, 25 (75.76%) received ssCART-19 cell therapy as a third or later salvage treatment, 26 (78.79%) were treated with other approaches for more than half a year, and 11 (33.33%) received bone marrow transplantation before ssCART-19 cell treatment. Nine (23.27%) patients were diagnosed with
Philadelphia chromosome (Ph)-positive ALL, 28 (84.85%) relapsed before ssCART-19 cell treatment, 1 (3.03%) had a TP53 gene mutation, and 11 (33.33%) were evaluated as poor cytogenetic risk patients.
Among the 45 patients in the high tumour burden group, 30 (66.67%) received ssCART-19 cell therapy as a third or later salvage treatment, 28 (62.22%) were treated with other approaches for more than half a year, and 8 (17.78%) received bone marrow transplantation before ssCART-19 cell treatment. Five (11.11%) patients were diagnosed with Ph-positive ALL, 29 (64.44%) relapsed before ssCART-19 cell treatment, 2 (4.44%) had a TP53 gene mutation, and 13 (28.89%) were evaluated as poor cytogenetic risk patients. Table 1 includes additional baseline patient information.
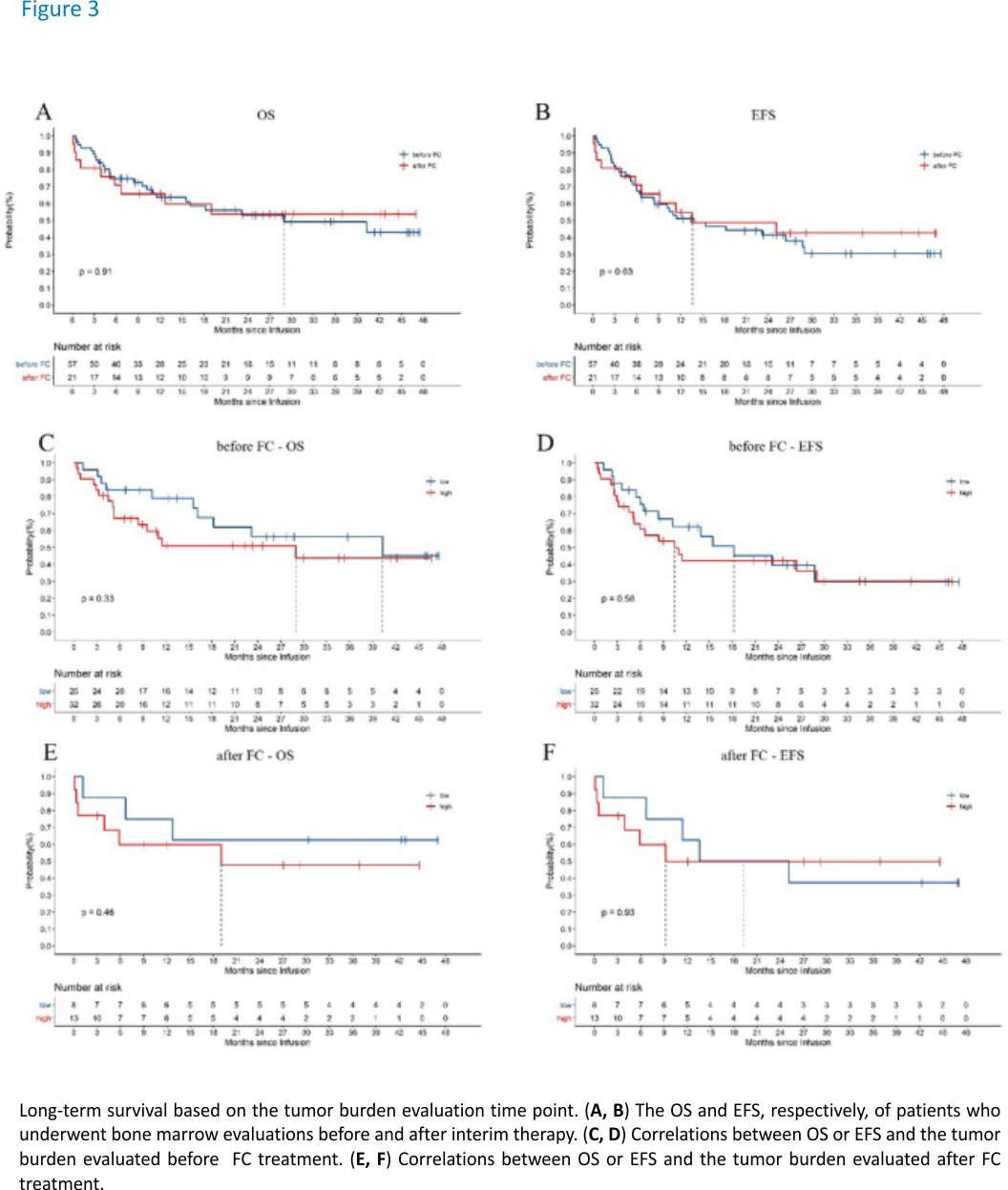
It is worth noting that the time of tumour burden evaluation in this study was not completely uniform. The evaluation time points were distributed before and after FC treatment. Among the study cohort, the tumour burdens of 25 (75.76%) people in the low tumour burden group were evaluated prior to FC treatment, while those of 32 (71.11%) people in the high tumour burden group were evaluated before FC treatment. According to the results of this study, the time point of tumour burden assessment had no significant effect on the long-term efficacy (Fig. 3).
Response rates and long-term survival
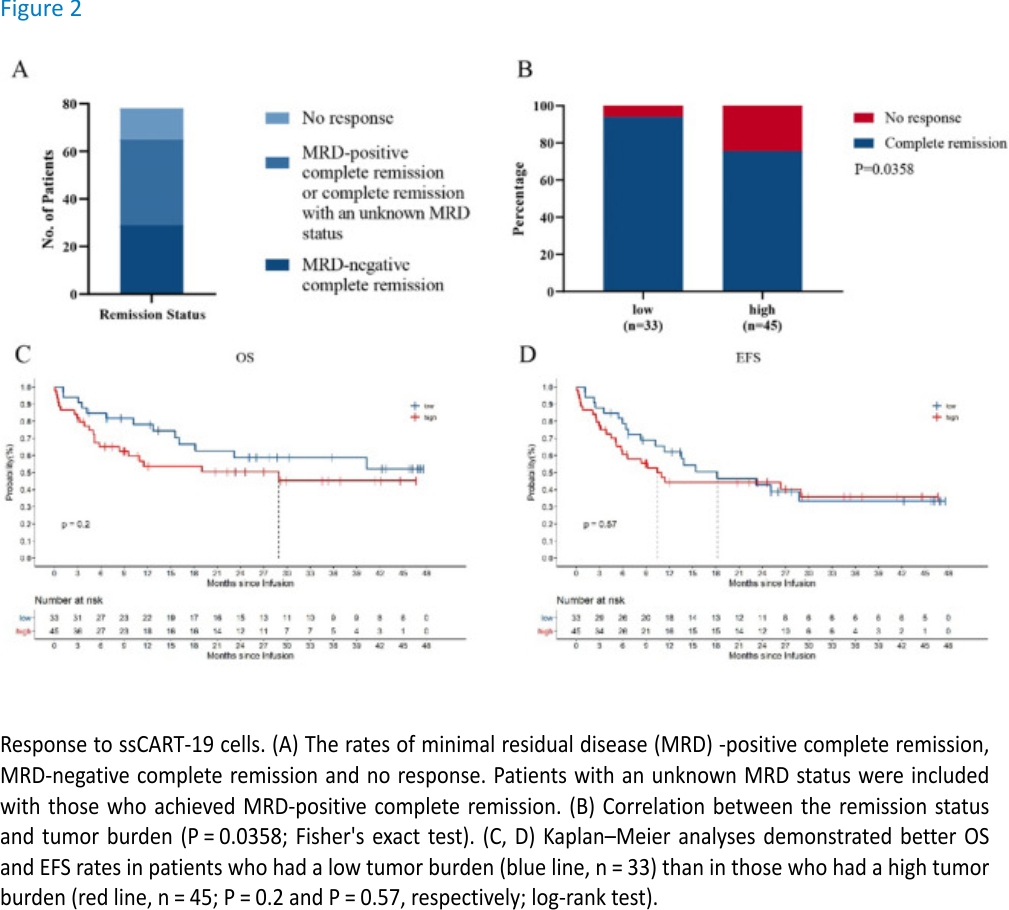
Among 78 patients, 29 achieved MRD-negative CR (37.18%), 36 achieved MRD-positive or MRD status-unknown CR (46.15%), and 13 patients had no response (NR) (16.67%). The overall CR rate reached 83.33% (Fig. 2A).
Analysis of the high and low tumour burden groups revealed that the CR rate was significantly correlated with the tumour burden. The CR rate of the low tumour burden group was significantly higher than that of the high tumour burden group (P = 0.0358, Fig. 2B). Analysis of the long-term survival data revealed that the long-term efficacy was also related to the tumour burden. Although the difference was not significant, the OS of patients in the low tumour burden group was better than that of patients in the high tumour burden group, and the EFS within 21 months of patients in the low tumour burden group was better than that of patients in the high tumour burden (Fig. 2C, D).
The time points of bone biopsy to assess the patients’ tumour burdens were not completely uniform due to the clinical specificities. Therefore, patient data were obtained from tumour burdens measured both before and after FC treatment in the study. To explore whether this difference affected the statistical results, we conducted grouping statistical analysis according to whether the tumour burden was assessed before or after FC treatment. According to our statistical results, the time point of tumour burden assessment did not affect the statistical results regarding OS and EFS after ssCART-19 cell treatment (Fig. 3A, B). Additionally, the tumour burden groups were further evaluated under this stratification, and the OS and EFS trends were consistent with those shown in Fig. 2C, D for the tumour burdens obtained both before and after FC treatment (Fig. 3C, F). These results suggest the tumour burden measurements both before and after FC treatment can be used as a reference in the clinic.
Safety analysis
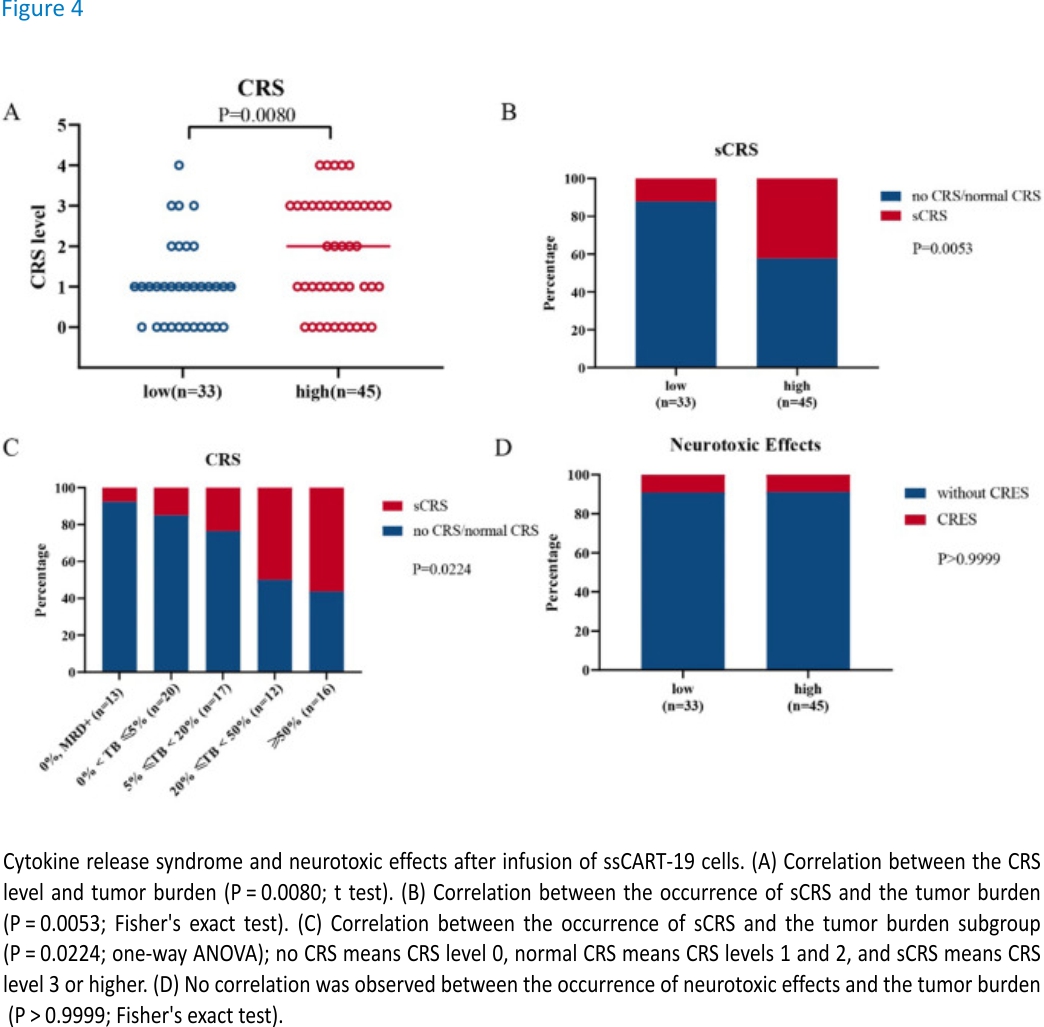
Statistical analysis showed a significant positive correlation between the tumour burden and the level of CRS response during treatment, which was basically consistent with the conclusion reported by Davila ML19. After mapping the CRS response level and the tumour burden groupings, the high tumour burden group had an increased CRS response and a higher CRS response level, thereby also indicating a higher incidence of sCRS, than the low tumour burden group (Fig. 4A, B). According to the specific tumor burden assessments, the patients were further divided into 5 subgroups based on their tumor burden measurements: (a) 0% tumor burden with MRD positivity; (b) 0% < tumor burden < 5%; (c) 5% ≤tumor burden < 20%; (d) 20% ≤tumor burden < 50%; and e) tumor burden ≥50%. The increasing tumour burden gradient in the tumour burden subgroups reflected an obvious correlation with sCRS and could therefore provide a reference for the clinical prevention of sCRS (Fig. 4C). There was no difference in neurotoxicity among the groups. It is worth emphasizing that ssCART-19 cells are suitable for the treatment of central nervous system leukaemia. This lack of a difference among groups might be related to the intrinsic characteristics of ssCART-19 cells, which could reduce neurotoxicity and improve safety (Fig. 4D).
Cytokine release
We conducted a more in-depth analysis of patients’ blood samples to further explore the causes of the phenomenon resulting from the clinical treatment described above.
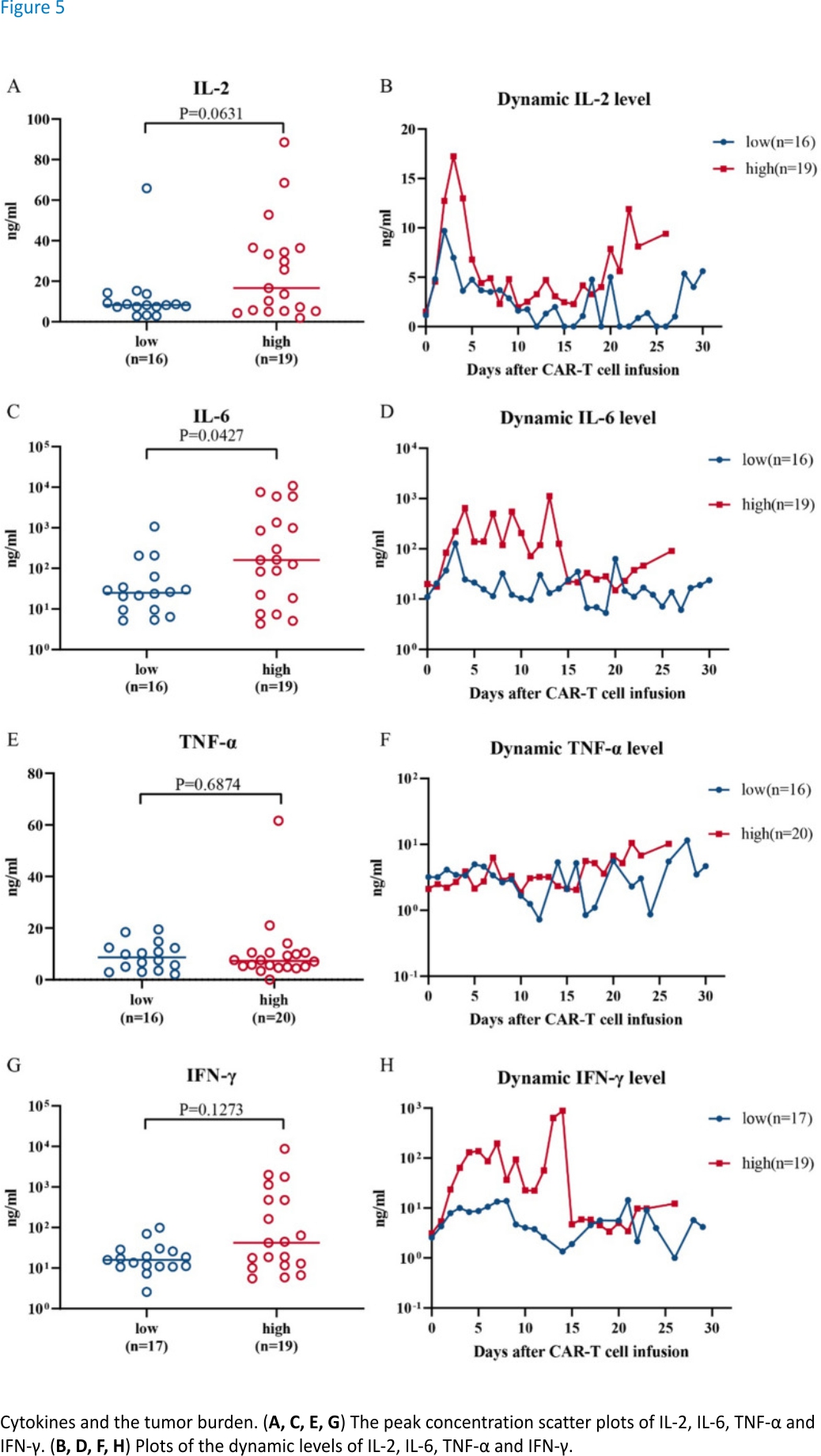
We studied the CRS response according to the protocol established by Marco L. Davila4 by investigating four cytokines that are potentially related to CRS: IL-2, IL-6, TNF-α and IFN-γ. A higher level of only the cytokine IL-6 was significantly correlated with the tumour burden (Fig. 5A, C, E, G). The amount of IL-6 released in the high tumour burden group was significantly higher than that released in the low tumour burden group (Fig. 5C, P = 0.0427). IL-6 is closely related to the CRS response22, suggesting that it is the main factor underlying the high sCRS response rate in the high tumour burden group. Based on the dynamic results for these four cytokines, TNF-α, IL-2, IL-6 and IFN-γ were secreted at higher levels in the high tumour burden group than in the low tumour burden group, but the differences were not obvious (Fig. 5B, D, F, H). This result suggests that patients in the high tumour burden group had a higher safety risk during the treatment process than those in the low tumour burden group.
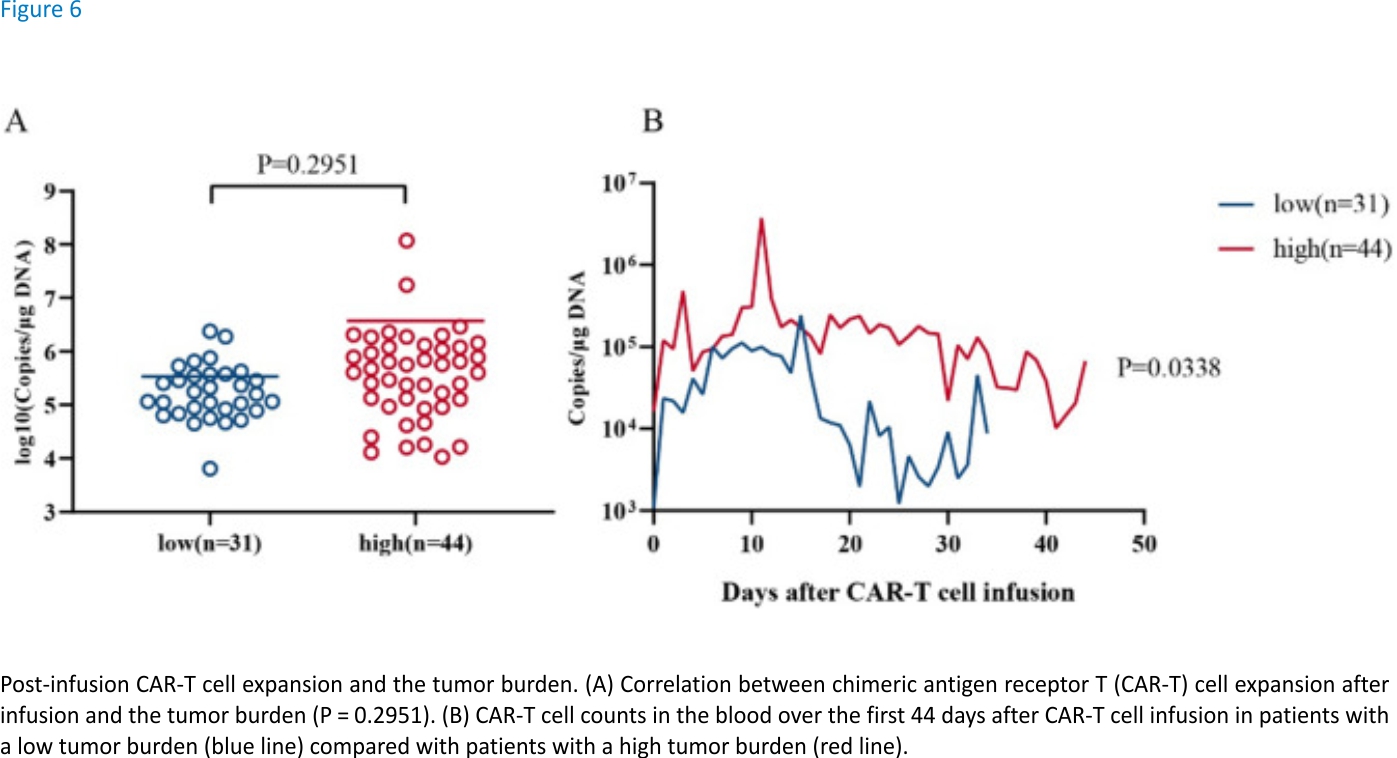
Correlation between the tumour burden and post-infusion CAR-T cell expansion
No significant correlation between the peak CAR-T cell expansion and the tumour burden was observed, suggesting that ssCART-19 cells are mild and stable in vivo. Although the average peak copy number of the high tumour burden group was higher than that of the low tumour burden group (Fig. 6A), the CAR-T cells did not overreact due to a large number of target antigens in the high tumour burden group, which improved the safety of the treatment. This result might also explain why the CR rate was significantly lower in the high tumour burden group than in the low tumour burden group.
CAR-T cell dynamics were plotted on a curve and analysed using a paired t-test. Although the copy numbers were assessed at different times, the overall statistical analyses showed significant differences (Fig. 6B). This analysis showed that patients with different tumour burdens responded differentially to ssCART-19 cell therapy, but the responses were not overly intense, thereby ensuring effective treatment while taking safety into account.
Discussion
CAR-T cell therapy triggers a cellular immune response via the specific activation of CAR-T cells by specific antigens on target cells. Therefore, the number of target cells affects the degree of CAR-T cell stimulation and the intensity of the immune response. B-ALL is a type of haematological tumour, and the tumour burden is equivalent to the target cell load. Therefore, the B-ALL tumour burden is one of the main factors that determine the strength of the bodily CAR-T cell-target cell-mediated cellular immune response and the performance and clinical efficacy of CAR-T cells during clinical treatment. The CAR-T cell treatment of B-ALL in patients with a high tumour burden is more likely to cause sCRS than that of patients with a low tumour burden6,19,20,21. sCRS is a systemic inflammatory response to CAR-T cell treatment caused by the release of cytokines from infused CAR-T cells and can lead to widespread reversible organ dysfunction26. Clear conclusions have not been drawn from the current research on whether the tumour burden affects the efficacy of CAR-T cells. Park, J. H concluded that patients with a high disease burden had shorter long-term survival than those with a low disease burden17, while Brentjens, R. J reported that the tumour burden was not correlated with clinical efficacy21. These differential results may be related to the specific treatment plans and number of clinical cases. For the study of Park, J. H, the different treatment processes for patients might have a significant impact on the clinical data. Firstly, 53 patients enrolled in the study, among them 43 patients received cyclophosphamide (Cy) conditioning chemotherapy before CAR-T infusion, and another 10 patients received Fludarabine + Cy chemotherapy. Secondly, the CAR-T infusion dosage was also different, 33 patients received different dosages of CAR-T cells. Some patients with a higher tumour burden received a lower dose of 1 × 106 19-28z CAR-T cells/kg. Some patients with a lower tumour burden received a higher dose of 3 × 106 19-28z CAR-T cells/kg. For the study of Brentjens, R. J, only 5 patients were treated during the study, from a statistical point of view, it is hard to obtain an accurate conclusion with this limited number of study subjects. Because CRS often occurs only during CAR-T cell treatment, the correlation between the tumour burden and CRS is easily studied. In contrast, drawing effective conclusions from the correlations of the tumour burden with EFS and OS often requires a sufficient number of patients and a long follow-up time. Therefore, a more systematic and reasonable approach to analysing clinical research data is critical and necessary.
In this study, 78 patients were treated with ssCART-19 cells and we systematically evaluated baseline parameters in the study participants in a standardized manner prior to the statistical analysis to ensure that the baseline data were consistent and that the various tumours burdens analyses were as reliable as possible. At the same time, we followed a consistent ssCART-19 treatment process including the conditioning chemotherapy, CAR-T dosage to reduce the impacts of factors other than the tumour burden.
Based on the above research and our research results, we conclude that ssCART-19 cells have good curative effects and that the CR rate is significantly related to the tumour burden. The OS and EFS results demonstrated that patients with a low tumour burden benefited more than those with a high tumour burden. Park, J. H17 reportedly observed no significant correlation between the tumour burden and the CR rate, but the tumour burden did significantly affect the OS and EFS. Although the conclusions of the two studies are not completely consistent, the overall efficacy (both CR rate and OS/EFS) trends are the same, and despite the significant differences, both studies suggest that a lower tumour burden leads to a higher CR rate and better OS/EFS. Furthermore, this conclusion is basically consistent with our previous study27. The differential conclusions may be due to the different treatment plans utilized in the two studies. The pretreatment chemotherapy regimens were different for the patients in the high and low tumour burden groups, and the CAR-T cell doses were also different. These differences can obviously significantly impact the treatment effects15. Brentjens, R. J reported that the tumour burden was not related to clinical efficacy21. We believe that his study did not include a sufficient number of patients, thereby leading to a lack of sufficient data to draw definitive conclusions.
The impact of the tumour burden on the safety of the treatment process observed herein is consistent with the previous reports6,19,20,21. We also found that the incidence of sCRS in patients with a high tumour burden was significantly higher than that in patients with a low tumour burden, indicating a lower safety risk of CAR-T cell therapy in patients with a low tumour burden. Additionally, we found a significant correlation between the IL-6 peak and tumour burden, which potentially explains why patients with a high tumour burden are more likely to develop sCRS. Based on this, we also further divided the patients into 5 subgroups according to their tumour burden, and the CRS reaction became increasingly severe as the tumour burden increased. This finding has not yet been reported and may provide a more accurate clinical reference which may serve as an important early warning and estimated reference value for the occurrence of sCRS risk during clinical CAR-T cell treatment. In addition, we statistically analysed the number of adverse events during the clinical treatment process, and the results were positively correlated (Fig. S2), suggesting that the relationship between adverse events and the tumour burden is basically consistent with that between CRS and the tumour burden. Laboratory indicators such as red blood cells, haemoglobin, lymphocyte percentage, C-reactive protein, ferritin, albumin, and γ-glutamyl transpeptidase levels are also correlated with the tumour burden (Fig. S3). Similarly, Pan, J found that patients had a fever and elevated ALT/AST levels and that the incidence of hypoxemia and coagulopathy were potentially related to leukaemia burden14.
We also herein investigated the pharmacokinetics of CAR-T cells in r/r B-ALL patients with varying tumour burdens, and the CAR-T cell expansion peak was not significantly correlated with the tumour burden. Therefore, ssCART-19 cells are mild and stable in vivo. Although the number of CAR-T cells in the high tumour burden group was higher than that in the low tumour burden group, the cells did not overreact even with the high tumour burden and more target antigens, demonstrating the improved safety of ssCART-19 cells. This result might also explain why the CR rate in the high tumour burden group was significantly lower than that in the low tumour burden group. A dynamic CAR-T cell curve was analysed using a paired t-test. Although the copy numbers were determined at different time points, the overall statistical results showed significant differences. This result demonstrates that while ssCART-19 cells prompt differential responses in patients with different tumour burdens, the response is not overly intense, thereby ensuring effective treatment while considering safety. Unlike other reports10,28,29, this study did not identify a correlation between CAR-T cell pharmacokinetics and the severity of CRS (Fig. S4). These results suggest that the treatment of patients with a high tumour burden is not as safe as that of patients with a low tumour burden, which may not simply be related to the number of CAR-T cells.
This study revealed that the tumour burden is a significantly important reference for the efficacy and safety of CAR-T cell treatment. Before CAR-T cell infusion, the tumour burden should be reduced to the greatest extent possible to improve the treatment effect and reduce the safety risk. In addition to conventional chemotherapy, chemotherapy combined with radiotherapy can potentially be utilized to improve the treatment effect, thereby reducing the tumour burden. For example, we have attempted to use chemotherapy and radiotherapy to reduce the high burden in patients with diffuse large B-cell lymphoma (DLBCL) before CAR-T cell treatment (clinical trial number NCT03196830) and found that radiotherapy is highly recommended before CAR-T cell treatment for R/R DLBCL patients30.
For extremely refractory patients, other methods to reduce the tumour burden may not be effective, and clinicians can consider adjusting the treatment plan to improve the treatment effect and reduce the safety risk. For example, Memorial Sloan-Kettering Cancer Center (MSKCC) and Fred Hutchinson Cancer Research Center (FHCRC) adopt risk-adapted CAR-T cell dosing strategies to choose the best dose for balancing efficacy and safety1,12,19,21. Most of these centres use a “split dose” regimen to reduce the clinical risk, and patients may benefit more when this strategy is utilized.
The impacts of other factors on survival were evaluated when assessing the patients’ baseline information in this study, but no significant correlations were found (data not shown). Whether the tumour burden assessments at different time points, such as before and after FC pretreatment, have differential effects on the CAR-T cell treatment response, severe CRS, EFS, and OS were previously unknown. However, this study showed that under uniform FC pretreatment conditions, assessing the tumour burdens before and after FC treatment did not affect the final statistical results (Fig. 3; Fig. S1). This provides flexibility for clinicians to assess the benefits of CAR-T cell clinical treatment when tumour burden detection at different time points is required.
Finally, the sample size may not be large enough after more subgroups are divided, therefore, more case data are needed to consolidate our research conclusions. And with the more related factors and more types of cytokines related to the CRS reported, the cytokines and biochemistry marker reported in this study may not be enough. Further studies may require more testing items.
Conclusion
The CAR-T cell treatment of r/r B-ALL patients with a low tumour burden has an obviously higher CR rate than that of patients with a high tumour burden. The time point of tumour burden assessment does not affect the predictions of CR rate, survival time and safety (incidence of sCRS) based on the tumour burden. When making an overall CAR-T cell treatment plan, minimizing the tumour burden may become an important goal. The results of this study provide an important basis for formulating a reasonable plan for the CAR-T cell treatment of r/r B-ALL to obtain the greatest benefits. They may also provide an important basis for rationally formulating an overall CAR-T cell treatment plan for other cancers to obtain greater benefits.
Data availability
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We thank Israth Jahan Tuhin and Masuma Akter Monty for revising the manuscript.
Funding
This study is supported by grants from the National Natural Science Foundation of China (81872812, 82073800, 81970138), Translational Research Grant of NCRCH (grant No. 2020ZKMB05), Jiangsu Province “333” project and Gusu Key Medical Talent Program (grant No. GSWS2019007), Social Development Project of the Science and Technology Department of Jiangsu (Grant No. BE2021649).
Author information
These authors contributed equally: Minghao Li, Sheng-Li Xue and Xiaowen Tang.
Affiliations
Institute of Biomedical Engineering and Technology, Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, NO, 3663 North Zhongshan Road, Shanghai, 200065, China
Minghao Li, Nan Xu, Jingwen Tan, Zhiqiang Yan & Lei Yu
National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Suzhou, China
Sheng-Li Xue, Xiaowen Tang, Suning Chen, Yue Han, Huiying Qiu, Miao Miao, Yang Xu, Jia Chen & Depei Wu
Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, China
Sheng-Li Xue, Xiaowen Tang, Suning Chen, Yue Han, Huiying Qiu, Miao Miao, Yang Xu, Jia Chen & Depei Wu
Shanghai Unicar-Therapy Bio-Medicine Technology Co., Ltd, No 1525 Minqiang Road, Shanghai, 201612, China
Liqing Kang, Zhou Yu & Xiaoyan Lou
Institute of Intelligent System and Bioinformatics, College of Intelligent Systems Science and Engineering, Harbin Engineering University, Harbin, 150001, Heilongjiang, China
Jiayu Xu & Weixing Feng
Contributions
L.Y., D.P.W., and M.H.L. contributed to the concept development and study design and wrote the manuscript. X.W.T., S.L.X. implement clinical trials and evaluate patients’ efficacy and safety, J.Y.X. collects data and analyze the data statistically. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Depei Wu or Lei Yu.
References
1. Frey, N. V. et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukaemia. J. Clin. Oncol. 38, 415–422. https://doi. org/10.1200/JCO.19. 01892 (2020).
2. Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukaemia. N. Engl. J. Med. 378, 439–448. https://doi.org/ 10.1056/NEJMoa1709866 (2018).
3. Kenderian, S. S., Porter, D. L. & Gill, S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: How not to put the CART before the horse. Biol. Blood Marrow Transpl. 23, 235–246. https://doi.org/10.1016/j.bbmt. 2016.09.002 (2017).
4. Curran, K. J. et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134, 2361–2368. https://doi.org/10.1182/ blood.2019001641 (2019).
5. Kotch, C., Barrett, D. & Teachey, D. T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert. Rev. Clin. Immunol. 15, 813–822. https://doi.org/ 10.1080/ 1744666X.2019.1629904 (2019).
6. Hay, K. A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 183, 364–374. https://doi.org /10.1111/bjh.15644 (2018).
7. Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukaemia. Cancer Discov. 6, 664–679. https://doi.org/10.1158/2159-8290.CD-16-0040 (2016).
8. Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738. https://doi.org/10.1038/s41591-018-0041-7 (2018).
9. Turtle, C. J. et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8, 355ra116. https://doi.org/10.1126/scitranslmed.aaf8621 (2016).
10. Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukaemia. Sci Transl Med 7, 303ra139. https://doi.org/10.1126/scitranslmed.aac5415 (2015).
11. Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukaemia. N. Engl. J. Med. 371, 1507–1517. https://doi.org/10.1056/ NEJMoa1407222 (2014).
12. Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138. https://doi.org/10.1172/JCI85309 (2016).
13. Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549. https://doi.org/10.1200/JCO.2014. 56.2025 (2015).
14. Pan, J. et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractories or relapsed B acute lymphoblastic leukaemia patients. Leukaemia 31, 2587– 2593. https://doi. org/10.1038/ leu.2017. 145 (2017).
15. Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukaemia. Nat. Med. 24, 563–571. https://doi. org/10.1038/s41591-018-0010-1 (2018).
16. Qin, J. S. et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J. Immunother. 43, 107–120. https://doi.org/10.1097/CJI. 0000000000000307 (2020).
17. Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459. https://doi.org/10. 1056/NEJMoa17099 19 (2018).
18. Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385, 517–528. https://doi.org /10.1016/ S0140-6736(14)61403-3 (2015).
19. Davila, M. L. et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6, 224ra225. https://doi.org/10.1126/scitranslmed. 3008226 (2014).
20. Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306. https://doi.org/10.1182/blood-2017-06-793141 (2017).
21. Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukaemia. Sci. Transl. Med. 5, 177ra138. https://doi.org/10.1126/scitranslmed.3005930 (2013).
22. Kang, L. et al. Interleukin-6-knockdown of chimeric antigen receptor-modified T cells significantly reduces IL-6 release from monocytes. Exp. Hematol.Oncol.9,11. https://doi.org/10. 1186/s40164-020-00166-2 (2020).
23. Ramos, C. A. et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J. Clin. Invest. 126, 2588–2596. https://doi.org/10.1172 /JCI86000 (2016).
24. Kang, L. et al. Characterization of novel dual tandem CD19/BCMA chimeric antigen receptor T cells to potentially treat multiple myeloma. Biomark. Res. 8, 14. https://doi.org/10.1186/s40364-020-00192-6 (2020).
25. Hoelzer, D. et al. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.27,v69–v82. https://doi.org/10.1093/ annonc/mdw025 (2016).
26. Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 127, 3321–3330. https://doi.org/10.1182/blood-2016-04-703751 (2016).
27. Li, L. et al. Treatment response, survival, safety, and predictive factors to chimeric antigen receptor T cell therapy in Chinese relapsed or refractory B cell acute lymphoblast leukemia patients. Cell Death Dis. 11, 207. https://doi.org/10.1038/s41419-020-2388-1 (2020).
28. Mueller, K. T. et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukaemia and chronic lymphocytic leukaemia. Blood 130, 2317–2325. https://doi.org/ 10.1182/blood-2017-06-786129 (2017).
29. Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531 – 2544. https://doi.org/ 10.1056/NEJMoa 1707447 (2017).
30. Qu, C. et al. Radiation priming chimeric antigen receptor T-cell therapy in relapsed/ refractory diffuse large B-cell lymphoma with high tumour burden. J. Immunother 43, 32–37. https://doi.org/ 10.1097/CJI.0000000000000284 (2020).
Credits: Li M, Xue SL, Tang X, Xu J, Chen S, Han Y, Qiu H, Miao M, Xu N, Tan J, Kang L, Yu Z, Lou X, Xu Y, Chen J, Yan Z, Feng W, Wu D, Yu L. The differential effects of tumour burdens on predicting the net benefits of ssCART-19 cell treatment on r/r B-ALL patients. Sci Rep. 2022 Jan 10;12(1):378. doi: 10.1038/s41598 -021-04296-3. PMID: 35013456; PMCID: PMC8748521.