Yi-Bang Cheng, Lutgarde Thijs, Lucas S. Aparicio, Qi-Fang Huang, Fang-Fei Wei, Yu-Ling Yu, Jessica Barochiner, Chang-Sheng Sheng, Wen-Yi Yang, Teemu J. Niiranen, José Boggia, Zhen-Yu Zhang, Katarzyna Stolarz-Skrzypek, Natasza Gilis-Malinowska, Valérie Tikhonoff, Wiktoria Wojciechowska, Edoardo Casiglia, Krzysztof Narkiewicz, Jan Filipovský, Kalina Kawecka-Jaszcz, Ji-Guang Wang, Yan Li, Jan A. Staessen and the International Database of Central Arterial Properties for Risk Stratification
(IDCARS) Investigators
Correspondence to Jan A. Staessen, APPREMED, Leopoldstraat 59, BE-2800 Mechelen, Belgium, Email jan.staessen@appremed.org
Correspondence to Yan Li, Shanghai Institute of Hypertension, Ruijin Hospital, Shanghai Jiatong University School of Medicine, Ruijin 2nd Rd 197, Shanghai 200025, China, Email liyanshch@163.com
Abstract
Background:
Whether a cardiovascular risk is more tightly associated with central (cSBP) than brachial (bSBP) systolic pressure remains debated, because of their close correlation and uncertain thresholds to differentiate cSBP into normotension versus hypertension.
Methods:
In a person-level meta-analysis of the International Database of Central Arterial Properties for Risk Stratification (n=5576; 54.1% women; mean age 54.2 years), outcome-driven thresholds for cSBP were determined and whether the cross-classification of cSBP and bSBP improved risk stratification was explored. cSBP was tonometrically estimated from the radial pulse wave using SphygmoCor software.
Results:
Over 4.1 years (median), 255 composite cardiovascular endpoints occurred. In multivariable bootstrapped analyses, cSBP thresholds (in mm Hg) of 110.5 (95% CI, 109.1–111.8), 120.2 (119.4– 121.0), 130.0 (129.6–130.3), and 149.5 (148.4–150.5) generated 5-year cardiovascular risks equivalent to the American College of Cardiology/American Heart Association bSBP thresholds of 120, 130, 140, and 160. Applying 120/130 mm Hg as cSBP/ bSBP thresholds delineated concordant central and brachial normotension (43.1%) and hypertension (48.2%) versus isolated brachial hypertension (5.0%) and isolated central hypertension (3.7%). With concordant normotension as a reference, the multivariable hazard ratios for the cardiovascular endpoint were 1.30 (95% CI, 0.58–2.94) for isolated brachial hypertension, 2.28 (1.21–4.30) for isolated central hypertension, and 2.02 (1.41–2.91) for concordant hypertension. The increased cardiovascular risk associated with isolated central and concordant hypertension was paralleled by cerebrovascular endpoints with hazard ratios of 3.71 (1.37–10.06) and 2.60 (1.35–5.00), respectively.
Conclusions:
Irrespective of the brachial blood pressure status, central hypertension increased cardiovascular and cerebrovascular risk indicating the importance of controlling central hypertension.
Novelty and Relevance
What Is New?
In a person-level meta-analysis of the International Database of Central Arterial Properties for Risk Stratification, outcome-driven thresholds for central systolic blood pressure (cSBP) were derived, and risk stratification based on the cross-classification of cSBP with brachial SBP (bSBP) was investigated.
What Is Relevant?
- In multivariable bootstrapped analyses, cSBP thresholds (in mm Hg) of 110, 120, 130, and 150 generated 5-year cardiovascular risks equivalent to the American College of Cardiology/American Heart Association bSBP thresholds of 120, 130, 140, and 160.
- Applying 120/130 mm Hg as cSBP/bSBP thresholds delineated concordant central and brachial normotension (43.1%) and hypertension (48.2%) versus isolated brachial hypertension (5.0%) and isolated central hypertension (3.7%).
- With concordant normotension as a reference, the multivariable hazard ratios for the cardiovascular endpoint were 1.30 for isolated brachial hypertension, 2.28 for isolated central hypertension, and 2.02 for concordant hypertension.
Clinical/Pathophysiological Implications?
In the presence of brachial systolic normotension, central systolic hypertension increased cardiovascular and cerebrovascular risk. Women made up close to 70% of the patient with central systolic hypertension but normal bSBP. These findings highlight the role of cSBP in risk stratification, in particular in women, and the need to tailor antihypertensive drug treatment.
In clinical practice, blood pressure (BP) is routinely measured at the brachial artery. The anatomic proximity of the heart, brain, and kidney to the central arteries and growing insights1,2 into the role of arterial stiffening in cardiovascular disease led to the viewpoint that vascular risk must be more closely associated with central than brachial BP. However, the tighter association of cardiovascular risk with central compared with brachial BP remains controversial,3–6 mainly because of the strong correlation between central and brachial BP,5,6 as measured on a continuous scale.
A categorical approach might avoid the incongruities in the published associations of target organ damage4,5 or the incidence of adverse health outcomes3,6 with central compared with brachial BP. Central and brachial BPs might be categorized into normotensive versus hypertensive levels, allowing study participants to be cross-classified as being consistently or incoherently normotensive or hypertensive based on their central versus brachial BP.7–11 Previous studies that apply such an approach focused on the prevalence of central versus brachial hypertension 9,11 or related target organ damage cross-sectionally8,10 or total and cardiovascular mortality prospectively7 to central hypertension on top of brachial BP. The cross-classification method critically depends on the applied thresholds separating normotension from hypertension. The 2017 US guideline established new brachial BP thresholds .12 With regard to the central BP thresholds, diastolic BP being similar throughout the arterial tree,13 only 1 study derived and validated thresholds for central systolic BP against the long-term risk of mortality.7 In the current study, the International Database of Central Arterial Properties for Risk Stratification (IDCARS)6,14 was analyzed to establish an outcome-driven threshold for central systolic BP considering fatal as well as nonfatal cardiovascular endpoints and to explore whether the cross-classification approach added to risk stratification in the general population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Previous publications describe the construction of the IDCARS database in detail.6,14 The longitudinal studies extracted from the IDCARS data resource qualified for the current analysis, if the information on brachial and central BP and cardiovascular risk factors were available at baseline if the central BP had been tonometrically measured if follow-up included both fatal and nonfatal endpoints if study reports had been published in peer-reviewed articles and if the study participants were representative for a population. All studies complied with the Helsinki Declaration on research in humans15 and were approved by the competent Institutional Review Boards. Participants provided informed written consent. Before transfer to the coordinating office in Leuven, Belgium, the data were stripped from all personal identifiers, and if required by national legislations, additional ethical clearances were obtained. Study-specific information on the catchment areas, sampling strategies, timeframes of recruitment and follow-up, participation rates, and the related literature sources are available in Table S1 and in the published study protocol.14
BP Measurement
Brachial BP was measured immediately before the hemodynamic assessment after participants had rested for at least 5 minutes, up to 15 minutes, in the supine position, using standard mercury sphygmomanometers or validated oscillometric devices.14 Brachial BP was the average or the last of 2 consecutive readings. Estimates of central BP were calibrated on brachial systolic and diastolic BP and derived with the use of the SphygmoCor system.16 Details of the central BP estimation were described in the Expanded Methods in the Supplemental Material.
Ascertainment of End Points
The vital status of participants and the incidence of endpoints were ascertained from appropriate sources in each country. The primary endpoint was a composite cardiovascular outcome consisting of cardiovascular mortality and nonfatal endpoints, including death from ischemic heart disease, sudden death, nonfatal myocardial infarction, coronary revascularization, heart failure and fatal and nonfatal cerebrovascular endpoints. Secondary endpoints included total mortality, fatal and nonfatal cardiac endpoints, and fatal and nonfatal cerebrovascular endpoints. All endpoints were validated against hospital files or medical records held by primary care physicians or specialists. In all outcome analyses, only the first event within each category was considered.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4, maintenance level 5. Interpolation of missing values as described in the Expanded Methods in the Supplemental Material. 17 We compared means and incidence rates using the large-sample z test and proportions by the χ2 statistic, respectively.
We obtained diagnostic thresholds for central systolic BP in 5 steps, using a bootstrapped procedure18 with the risk associated with the office BP categories according to the 2017 American hypertension guideline as a reference standard.12 For the details of the bootstrapped procedure, please refer to the Expanded Methods in the Supplemental Material. After having established the central systolic BP thresholds, the incidence rates of endpoints were cross-classified by the presence of central and brachial systolic hypertension, irrespective of treatment status. Henceforth, normotension and hypertension refer to the systolic BP status, disregarding the intake of antihypertensive drugs at baseline. The incidence rates of endpoints were standardized for cohort, sex, and age group (≤50 versus >50 years) by the direct method. We computed 95% CIs of rates as R±1.96×√(R/T), where R and T are the rates and the denominator used to calculate the rate.19
In multivariable-adjusted Cox models, hazard ratios were expressed for patients with isolated central or brachial hypertension or central combined with brachial hypertension, using participants with central and brachial systolic normotension as reference. Cox models accounted for the cohort as a random effect and a propensity score generated by a logistic procedure with the LINK=GLOGIT option.20 We checked the proportional hazards assumption by the Kolmogorov-type supremum test. Statistical significance was a 2-sided probability of 0.05 or less.
Results
Characteristics of Participants
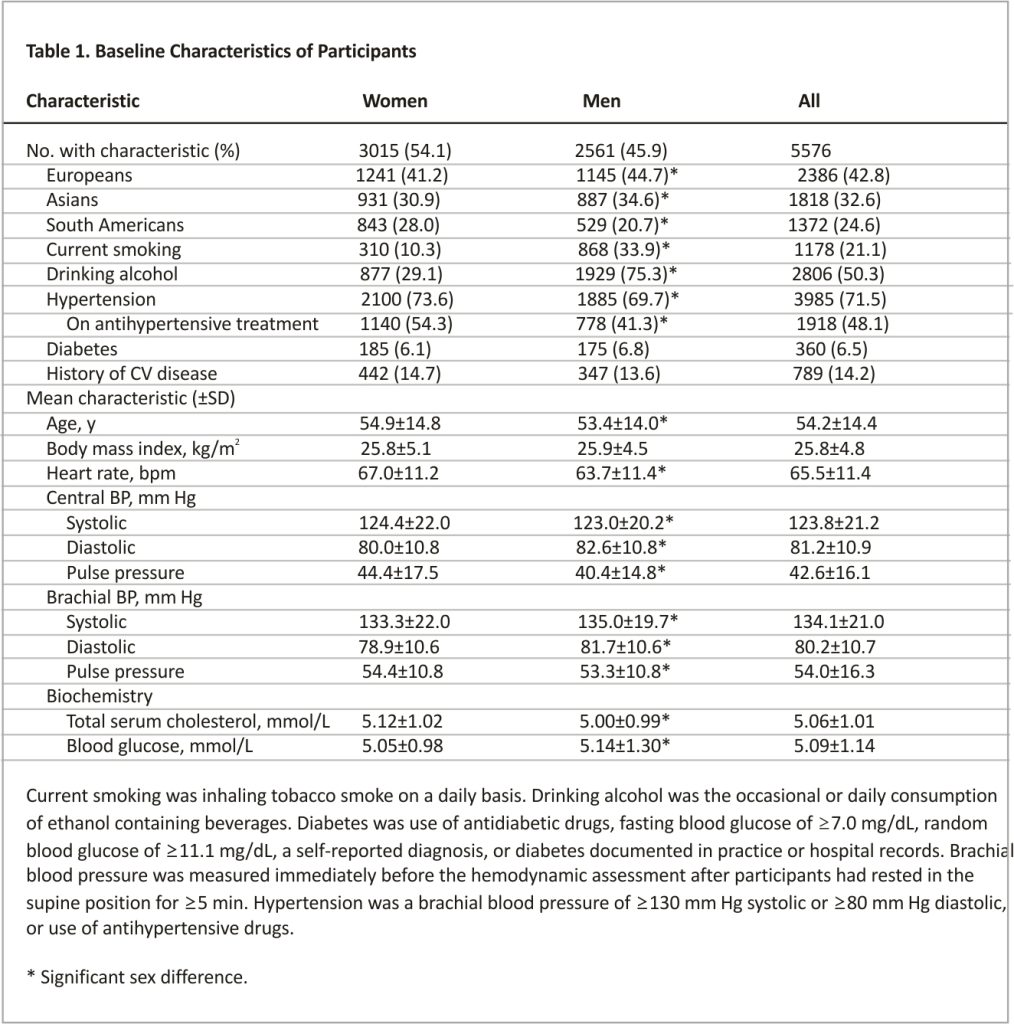
Of the 6650 participants from 9 cohorts, 1074 were excluded. The reasons for exclusion were described in the Expanded Results in the Supplemental Material. The number on interpolated values amounted to 23 (0.4%) for body mass index, 223 (4.0%) for smoking and 1171 (21.0%) for drinking, and 69 (1.2%) for history of cardiovascular disease.
In all participants, the mean age at enrollment was 54.2 years (Table 1). Central compared with brachial systolic BP was on average 10.3 mm Hg lower ([95% CI, 10.2–10.5]; P<0.001). Accordingly, central pulse pressure was also 11.4 mm Hg smaller ([95% CI, 11.2–11.5]; P<0.001) than brachial pulse pressure. However, there was large interindividual variability in the differences between brachial minus central systolic BP and between central minus brachial pulse pressure (Figure S1). The Pearson correlation coefficients between central and brachial systolic BP and between central and brachial pulse pressure were 0.97 (P<0.0001) and 0.95 (P<0.0001), respectively (Table S2).
Among all participants, 3985 had hypertension, 1918 were on antihypertensive treatment, and 1809 patients reported the information on antihypertensive drugs, of whom 392 (7.0%) and 1417 (25.4%) were taking a single agent or combination therapy, respectively. Drug classes taken were diuretics in 647 (16.2%) patients, β-blockers in 747 (18.8%), inhibitors of the renin-angiotensin system in 1181 (29.6%), and vasodilators in 831 (20.9%). Between-sex comparisons in characteristics (Table 1) were described in the Expanded Results in the Supplemental Material.
Thresholds for Central Systolic BP
In the overall study population, the median follow-up time was 4.1 years (5th–95th percentile interval, 2.2–12.1 years). Over 31 481 person-years, 255 participants experienced the primary cardiovascular endpoint (8.3 per 1000 person-years), 203 died (6.4 per 1000 person-years), and 164 had a cardiac endpoint (5.3 per 1000 person-years), and 89 had a cerebrovascular event (2.8 per 1000 person-years). Table S3 provides details on the components of the primary cardiovascular endpoint.
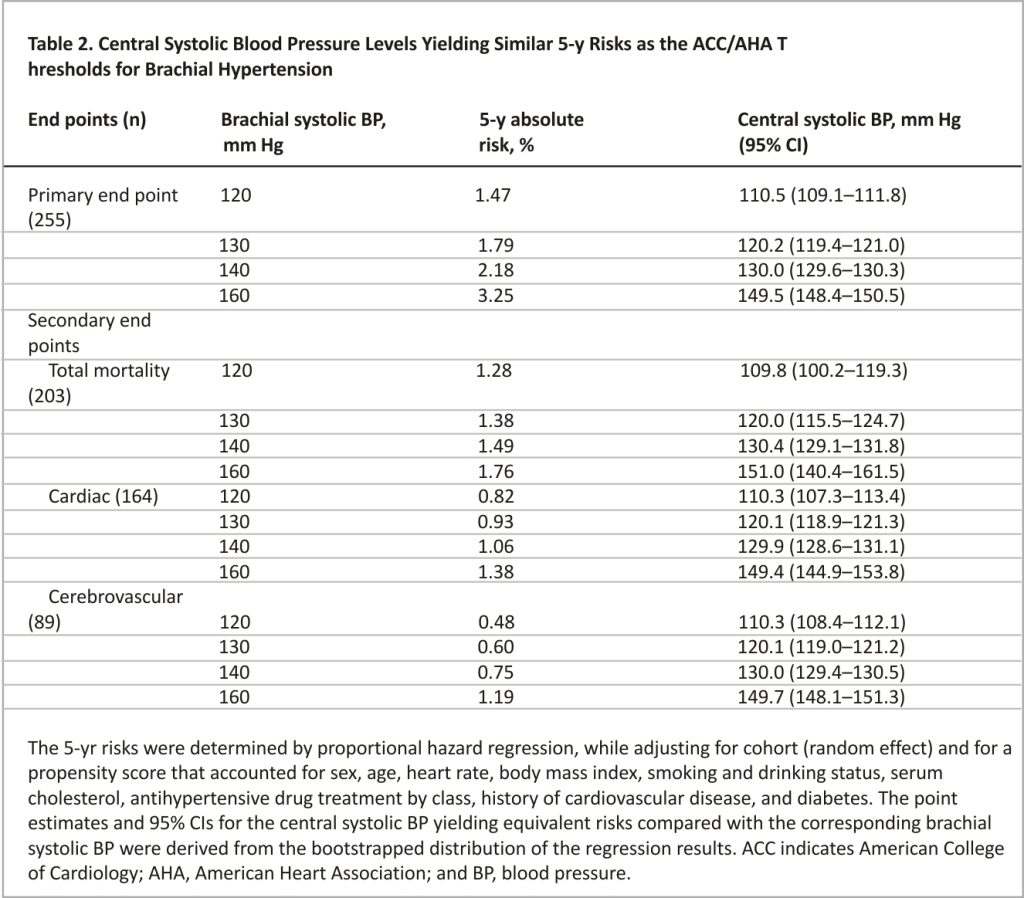
The thresholds of central systolic BP yielding a 5-year probability of experiencing the primary cardiovascular endpoint corresponding with the 5-year risk at the guideline-endorsed brachial BP thresholds of 120, 130, 140, and 160 mm Hg were determined by proportional hazard regression while adjusting for cohort (random effect) and a propensity score that accounted for sex, age, heart rate, body mass index, smoking and drinking status, serum total cholesterol, antihypertensive drug treatment by class, history of cardiovascular disease, and diabetes (Table 2). These central systolic BP thresholds were 110.5, 120.2, 130.0, and 149.5 mm Hg, respectively. The thresholds based on the full data set were similar to the means of the bootstraps. The central systolic BP thresholds for the secondary endpoints were similar to those derived for the primary cardiovascular endpoint (Table 2). In all these Cox models and all that follow later in this article, the proportional hazard regression assumption was met.
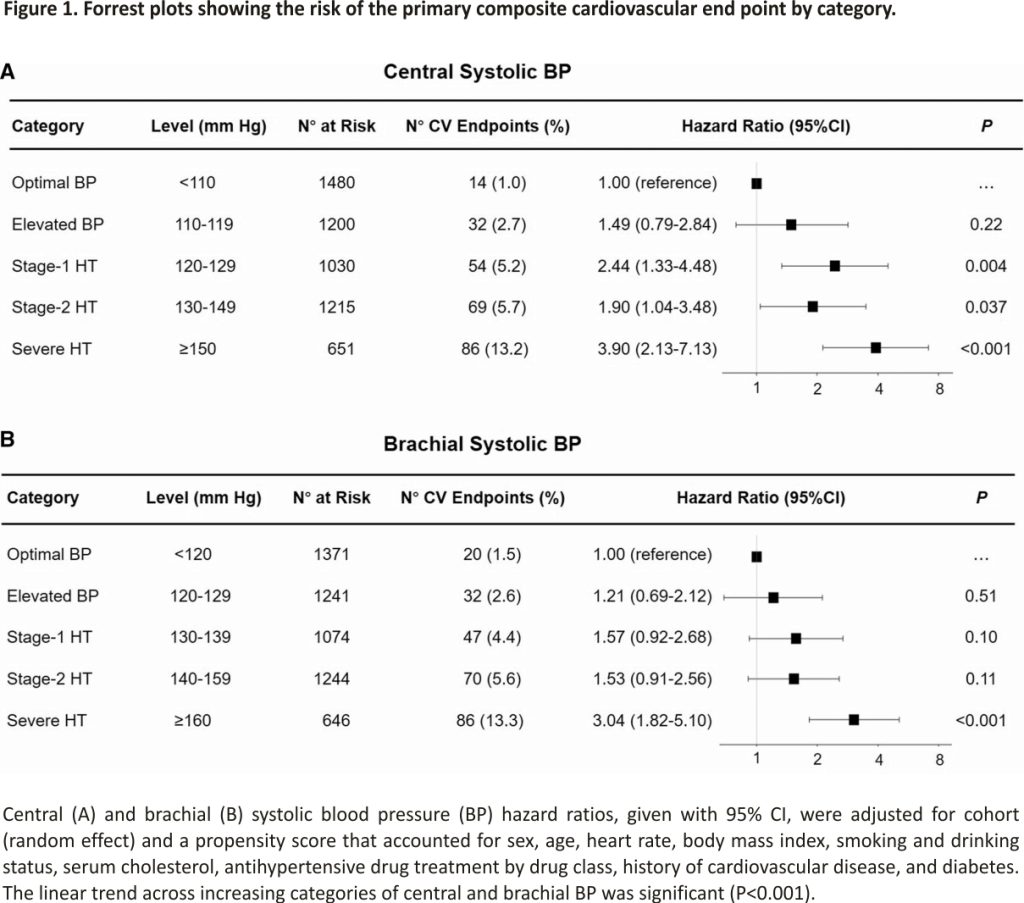
Sensitivity analyses from which 1918 participants on antihypertensive treatment at enrollment (Tables S4) or 789 with a history of cardiovascular disease (Table S5) were excluded produced consistent results. To obtain more easily recallable thresholds for central systolic BP, we rounded the point estimates obtained in Table 2 to an integer value ending in 0 or 5. These rounded thresholds indicating elevated BP, stage 1, stage 2 and severe hypertension based on central systolic BP were 110, 120, 130, and 150 mm Hg, respectively, consistent with the mean difference between central and brachial systolic BP (Table 1). With an increasing category of central or brachial systolic BP, the risk of the primary cardiovascular endpoint increased (Figure 1).
Cross-Classification of Central and Brachial Systolic Hypertension
The currently derived threshold for central systolic BP (120 mm Hg) and the American College of Cardiology/ American Heart Association threshold for brachial systolic BP (130 mm Hg) were applied for cross-classifying the IDCARS participants. In exploratory analyses, the number of primary cardiovascular endpoints amounted to 39/2403 (1.6%) in participants with concordant normotension, 20/486 (4.1%) in patients with discordant hypertension (central normotension combined with brachial hypertension or vice versa), and 196/2687 (7.3%) in patients with concordant hypertension, resulting in significantly increasing cohort-, sex- and age-standardized rates across the 3 groups of 2.66 (95% CI, 2.56–3.13), 8.33 (7.75–9.10), and 16.18 (15.81–16.83) endpoints per 1000 person-years, respectively (Figure S2). The trend in the primary cardiovascular endpoint was driven by cerebrovascular events, of which the numbers were 12/2403 (0.5%), 9/486 (1.9%), and 68/2687 (2.5%) in participants with concordant normotension and discordant and concordant hypertension, respectively; the corresponding rates expressed per 1000 person-years were 0.70 (0.68–1.18), 3.77 (3.51–4.25), and 5.40 (5.27–5.91; P for trend <0.001), respectively.
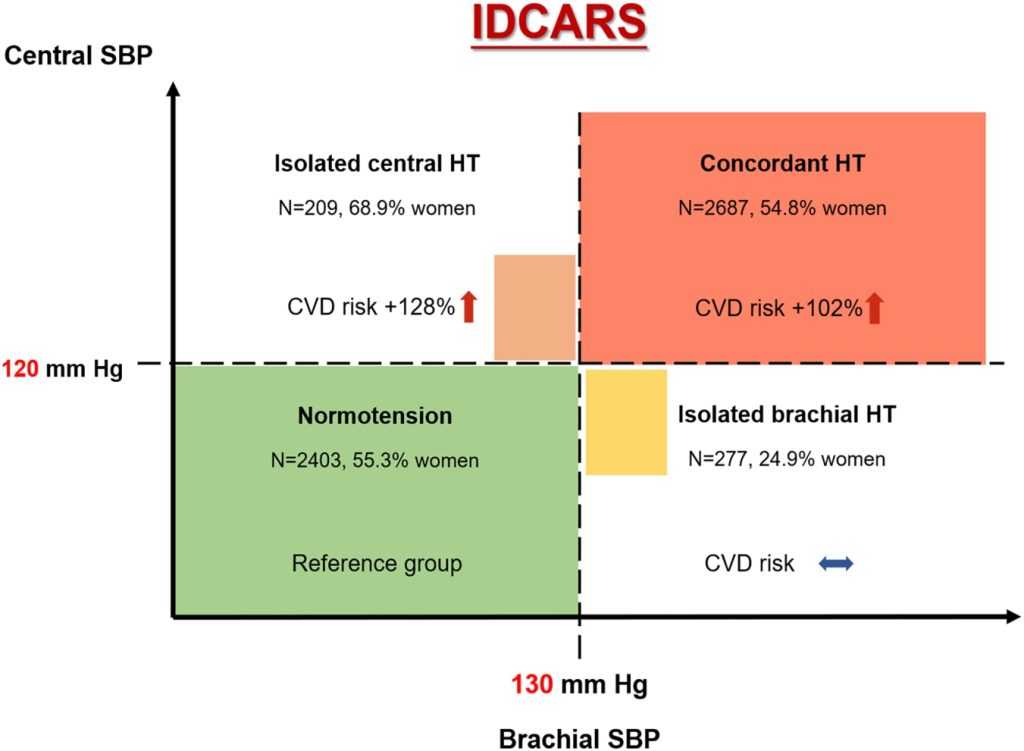
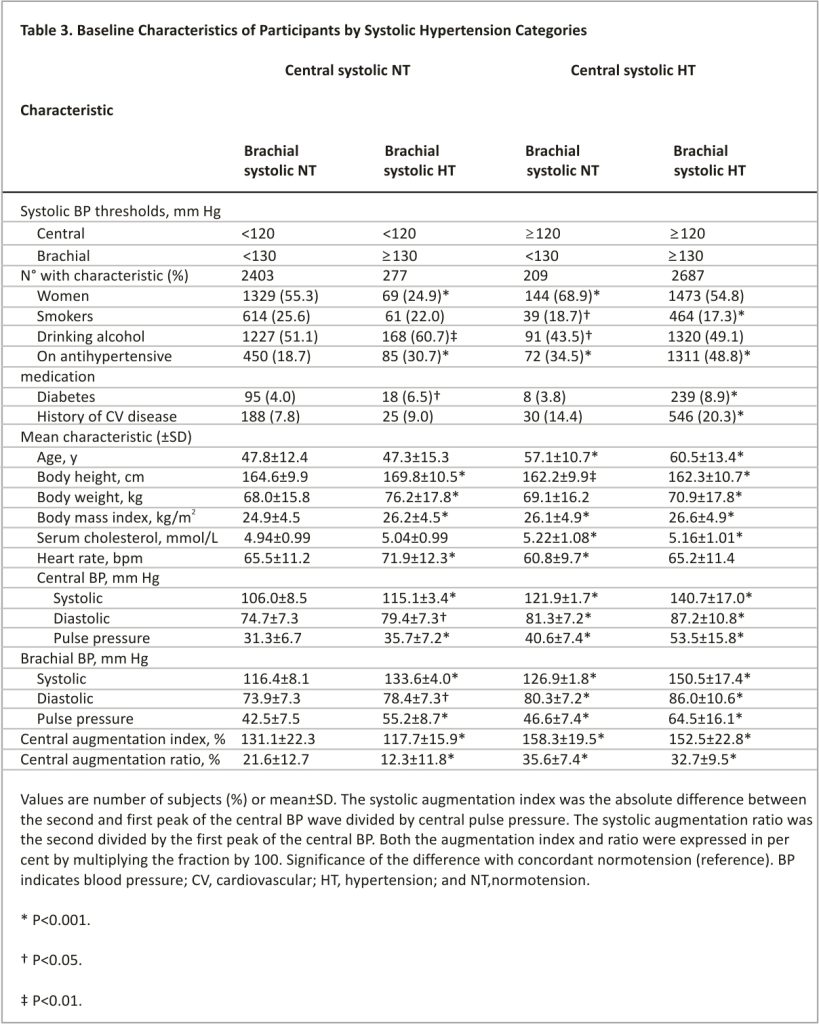
To clarify whether the risk in the patients with discordant hypertension was associated with central or brachial hypertension, the following next analyses were stratified into 4 groups, by subdividing the discordantly hypertensive group into patients with central normotension but brachial hypertension and patients with central hypertension but brachial normotension. Thus, the so-demarcated categories (Table 3) included concordant central and brachial systolic normotension (n=2403; 43.1% of the total IDCARS study population), isolated brachial hypertension (n=277; 5.0%), isolated central systolic hypertension (n=209; 3.7%), and concordant central and brachial hypertension (n=2687; 48.2%). Table S6 provides the distribution of the 4 groups in each cohort contributing to the present analysis.
Characteristics of the 4 Cross-Classified Groups
Compared with normotensive individuals, patients with isolated brachial hypertension were more likely to be male and drinkers, were taller and heavier, and had faster heart rate and less central augmentation. Patients with isolated central and concordant hypertension shared similarities in characteristics. They were older, had higher body mass index, serum total cholesterol, and central augmentation, and were more likely to have a history of cardiovascular disease or to be on antihypertensive drugs than subjects with concordant normotension (Table 3). Patients with isolated central hypertension were more likely to be female, had the slowest heart rate among the 4 groups (Table 3), and more frequently took β-blockers compared with the patients with isolated brachial hypertension (Table S7; P=0.008). Table S7 presents detailed information on the antihypertensive drugs taken at baseline in the 4 cross-classified groups and Figure S3 on the distribution of central and brachial pulse pressure in the 4 groups.
Absolute Risk by Cross-Classified Groups
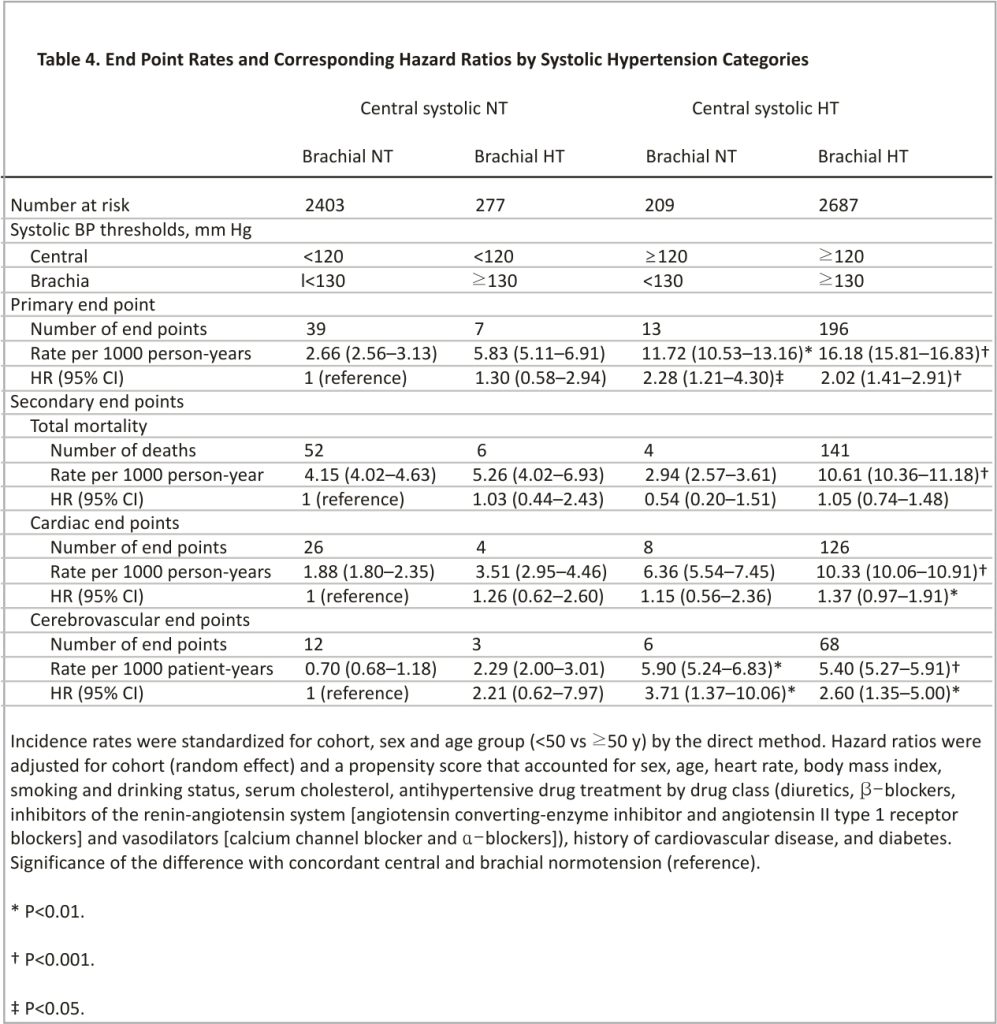
Compared with concordant central and brachial normotension, the cohort-, sex- and age-standardized incidence rates of all endpoints were higher in patients with concordant central and brachial hypertension (P<0.001; Table 4); the rates of the primary cardiovascular endpoint and cerebrovascular events were also significantly higher (P≤0.005) in patients with central hypertension in the presence of brachial normotension (Table 4).
Relative Risk by Cross-Classified Groups
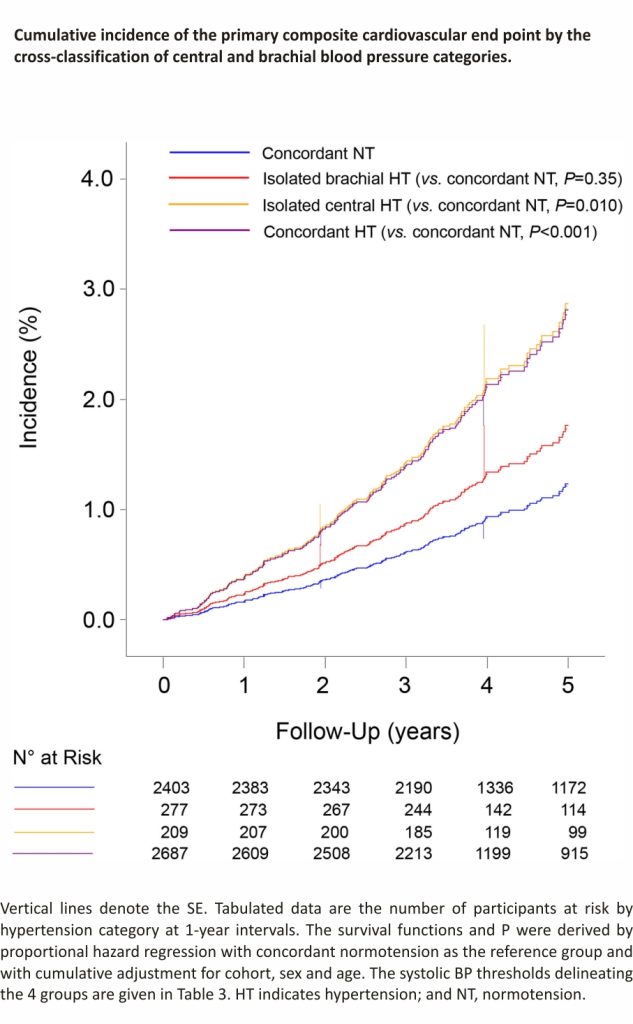
The cumulative incidence of the primary endpoint increased gradually from concordant central and brachial normotension over isolated brachial hypertension to isolated central hypertension and onward to concordant central and brachial hypertension (P<0.001) with no difference between isolated central hypertension and concordant hypertension (P=0.35; Figure 2). With concordant normotension as reference, the multivariable-adjusted hazard ratios were 1.30 (95% CI, 0.58– 2.94; P=0.52) for isolated brachial hypertension, 2.28 (1.21–4.30; P=0.011) for isolated central hypertension, and 2.02 (1.41–2.91; P<0.001) for concordant hypertension (Table 4). Sensitivity analyses excluding patients with central and brachial diastolic hypertension (≥80 mm Hg, n=2978, Table S8) or participants taking β-blockers at baseline (n=747, Table S9), or patients on antihypertensive drug treatment at enrollment (n=1918, Table S10) produced risk estimates, which confirmed the results presented in Table 4. In particular, in untreated participants, the incidence of the primary endpoints increased from concordant normotension over isolated brachial hypertension to isolated central hypertension and onwards to concordant hypertension (P for trend, 0.023). Among untreated participants, the hazard ratios were directionally similar compared with the main analysis (Table 4), but in view of the smaller number of events and people at risk, formal statistical significance was only reached for the primary and cerebrovascular endpoints among patients with concordant central and brachial systolic hypertension.
Discussion
The IDCARS cohort included community-dwelling participants, and representatives of the country, where they had been recruited and followed up. Central systolic BP and central pulse pressure were on average 10 mm Hg lower than their brachial counterparts (Table 1). However, there was large interindividual variability around these average differences in systolic amplification (Figure S1). This observation justified the derivation of thresholds for central systolic BP with a 95% CI around the point estimates based on the equivalence of risk with the established American College of Cardiology/American Heart Association diagnostic thresholds for brachial BP.12 The so-derived thresholds for central systolic BP, rounded to the closest integer were 110, 120, 130, and 150 mm Hg, respectively, for elevated BP, stage 1, stage 2, and severe hypertension. The gradual increase in fatal combined with nonfatal cardiovascular complications with higher categories of central and brachial BP provided the validation of this approach (Figure 1). The cross-classification of central hypertension (threshold 120 mm Hg) versus brachial hypertension (threshold, 130 mm Hg) demonstrated similar risks in patients with isolated brachial hypertension compared with concordant normotension (Figure 2; Table 4). Patients with isolated brachial hypertension, in the literature also referred to as spurious systolic hypertension, were predominantly tall men (Table 3) with no increased risk of adverse health outcomes (Table 4), a finding which is consistent with pulse wave dynamics and previous reports.21,22 However, patients with isolated central hypertension showed hazard ratios of fatal combined with nonfatal cardiovascular and cerebrovascular endpoints approaching the risks in concordantly hypertensive patients. In a previous IDCARS analysis,6 the associations of endpoints were similar for central and brachial systolic BP, because of the high correlation between both BP indexes (r=0.97). Our current observations generated new insights by identifying small groups without or with increased cardiovascular risk based on the cross-classification of central and brachial systolic hypertension, thereby illustrating the clinical utility of measuring both central and brachial systolic BP.
The reference values for arterial measurements collaboration analyzed 18 183 healthy people and 29 605 patients with one or more cardiovascular risk factors, including hypertension.23 All individuals were not on antihypertensive or lipid-lowering drug treatment and were free from cardiovascular disease and diabetes. In analyses stratified by the presence versus absence of cardiovascular risk factors, amplification decreased only slightly with age, whereas the overriding determinant of systolic amplification was sex, given that the difference between brachial minus central systolic BP was 6.6 mm Hg (95% CI, 5.8–7.4 mm Hg) less in women than men. In the normal population, the 90th percentiles for optimal, normal, and high-normal central systolic BPs were 110, 125, and 135 mm Hg in women and 111, 122, and 132 mm Hg in men.23 The currently proposed thresholds for central systolic BP were only stratified by brachial systolic BP, but sex and age were included in the propensity score used for their derivation. The rounded thresholds listed in Table 2 are therefore applicable, irrespective of sex facilitating their clinical application.
Only one previous study reported thresholds for central systolic BP based on adverse health outcomes.7 Cheng et al7 determined diagnostic thresholds for central systolic BP in a derivation cohort consisting of 1272 individuals followed up for a median of 15 years and replicated these thresholds in a test cohort comprising 2501 individuals with a median follow-up of 10 years. All study participants were untreated for hypertension. The thresholds for central systolic BP were generated using the same bootstrapped approach as in the current study with as objective to determine the central systolic BP levels that yielded the same risk of cardiovascular mortality as associated with brachial systolic BP levels of 120 mm Hg (optimal BP) and 140 mm Hg (hypertension). After rounding, the systolic threshold was 110 mm Hg for optimal BP and 130 mm Hg for hypertension. Compared with optimal BP, the risk of cardiovascular mortality increased significantly in patients with hypertension (hazard ratio, 3.08 [95% CI, 1.05–9.05]). The present study extends Cheng’s observations7 in a multiethnic and multicultural context by considering fatal combined with nonfatal endpoints as well as target organ-specific endpoints, such as cerebrovascular events. Thus, Cheng’s study7 and IDCARS provided mutually replicative findings with the same rounded thresholds for optimal and hypertensive levels of central systolic BP. Some differences between the 2 studies deserve to be highlighted. In the IDCARS analyses, diastolic BP was not considered and 48.1% of the IDCARS study population were on anti-hypertensive drug treatment at enrollment. Diastolic BP is similar throughout the arterial tree.13 Thus, in Cheng’s study, the outcome-driven thresholds for central diastolic BP yielding a risk of cardiovascular mortality equivalent to brachial diastolic BP levels of 80 and 90 mm Hg were 80.92 and 90.98 mm Hg, respectively. Sensitivity analyses of the IDCARS data addressed the issues of antihypertensive drugs (Table S4), specific treatment with β-blockers (Table S9), and diastolic hypertension (Table S8).
While the IDCARS database is a powerful resource, some limitations in its exploitation must also be acknowledged. First, a single type-124 central BP monitoring (SphygmoCor) was used for the noninvasive assessment of the central hemodynamic traits. The SphygmoCor algorithm preserves the systolic amplification as evidenced by the 10 mm Hg mean difference between the central and brachial arteries. Nevertheless, the accuracy of the SphygmoCor approach is vulnerable to errors in the measurement of brachial BP,25 which is needed for calibration, and it does also not account for pulse wave amplification from the brachial to the radial artery.26 However, as highlighted in the Methods, the quality control of the arterial phenotypes was rigorously standardized in IDCARS. In all but one cohort, the brachial BP used to calibrate the central pulse wave was obtained by automated oscillometric devices,14 which to a large extent excludes observer bias. While the use of a single type-1 system might be considered a strength in terms of standardization, it might also limit generalizability. However, as suggested by a previous meta-analysis,3 there is little device-dependent heterogeneity in the association of adverse health outcomes and central systolic BP. Second, the anthropometric characteristics, the period of recruitment, and the assessment of endpoint data differed between cohorts (Table S1). However, the present analyses were adjusted for the cohort as a random effect. By design, participant-level meta-analyses allow applying the same statistical methods to all contributing cohorts. Moreover, the diversity of the IDCARS cohorts strengthens the generalizability of our current results. Third, although the IDCARS participants currently analyzed were enrolled in 8 countries and 3 continents, the analyses did not include people younger than 30 years, because they did not contribute to the incidence of the primary endpoint. Furthermore, Blacks show a steeper relation of adverse health effects with both central and brachial systolic BP, for instance, illustrated for left ventricular hypertrophy in a Sub-Saharan cohort.27 Thus, the current observations cannot be extrapolated to people with Black ancestry. Fourth, risk factors and antihypertensive drug treatment were only quantified at enrollment, so analyses could not be adjusted for time-varying covariables. Finally, cross-classifying the IDCARS participants into 4 groups led to a small number of cardiovascular and cerebrovascular endpoints in the discordant groups (Table 4). We addressed this issue by implementing the multivariable adjustment by a propensity score. The 95% confidence interval of the hazard ratios expressing the relative risk of a cardiovascular or cerebrovascular endpoint in patients with central hypertension but brachial normotension compared with concordant normotension were not exceedingly large, suggesting that the risk estimates were relatively precise.
Perspectives
The patients with central systolic hypertension but brachial normotension are a minority, in IDCARS representing only 3.7% of the total study population. However, these patients include close to 70% of women (Table 3), in whom cardiovascular risk is often ignored28 and close to 20% of patients on treatment with β-blockers (Table S7). The CAFÉ (Conduit Artery Function Evaluation) study29 examined the impact of 2 different BP-lowering regimens (atenolol±thiazide -based versus amlodipine±perindopril -based therapy) on central aortic pressures as derived from the radial pulse wave by means of the SphygmoCor technology. Despite similar brachial systolic BPs between treatment groups (difference, 0.7 mm Hg [95% CI, −0.4 to 1.7]; P=0.20), there were substantial reductions in central aortic pressures with the amlodipine-based regimen, amounting to 4.3 mm Hg (95% CI, 3.3–5.4) for central systolic BP and 3.0 mm Hg (95% CI, 2.1–3.9) for central pulse pressure. Thus, the CAFE investigators generated important evidence that should guide clinical practice in patients with central systolic hypertension but brachial normotension. Clinicians should become aware that even in the presence of brachial normotension, an assessment of central systolic BP might help in risk stratification and optimizing antihypertensive drug treatment.
Article Information
Sources of Funding
Argentina: The Internal Medicine Service, Hospital Italiano de Buenos Aires, Buenos Aires; Belgium (Leuven): European Union (HEALTH-F7-305507 HOMAGE), European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), and European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT); Belgium (APPREMED): The Research Institute Alliance for the Promotion of Preventive Medicine (URL: www. appremed.org) received a nonbinding grant from OMRON Healthcare Co., Ltd., Kyoto, Japan; China: The National Natural Science Foundation of China (grants 81770455, 81970353, 82070432, 82070435, 91639203), the Ministry of Science and Technology (2018YFC1704902), Beijing, China, and by the Shanghai Commissions of Science and Technology (grants 19ZR1443300 and “Sailing Program” 19YF1441000), the Shanghai Shenkang Hospital Development Center (SHDC2020Cr10- 42B), and the Shanghai Municipal Health Commission (201940297, GWV-10.1-XK05 and a Grant for Leading Academics); Czech Republic: European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550) and Charles University Research Fund (project P36); Finland: Academy of Finland (grant 321351), Emil Aaltonen Foundation, the Finnish Foundation for Cardiovascular Research, and the Hospital District of South-Western Finland; Italy: European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550); Poland (Gdańsk): European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550); Poland (Kraków): European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007– 201550) and Foundation for Polish Science.
Nonstandard Abbreviations and Acronyms
ACC – American College of Cardiology
AHA – American Heart Association
BP – blood pressure
bSBP – brachial systolic blood pressure
CAFÉ – Conduit Artery Function Evaluation
cSBP – central systolic blood pressure
IDCARS – International Database of Central Arterial Properties for Risk Stratification
Disclosures
None.
References
1. O’Rourke MF. Influence of ventricular ejection on the relationship between central aortic and brachial pressure pulse in man.Cardiovasc Res. 1970; 4:291–300. doi: 10.1093 /cvr/ 4.3.291
2. Safar ME, Toto-Moukouo JJ, Bouthier JA, Asmar RE, Levenson JA, Simon AC, London GM. Arterial dynamics, cardiac hypertrophy, and antihypertensive treatment.Circulation. 1987; 75(1 Pt 2):I156–I161.
3. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis.Eur Heart J. 2010; 31:1865–1871. doi: 10.1093/eurheartj/ehq024
4. Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of Central Versus Brachial Blood Pressure with Target-Organ Damage: Systematic Review and Meta-Analysis. Hypertension. 2016; 67:183–190. doi: 10.1161/HYPERTENSIONAHA. 115.06066
5. Yang WY, Mujaj B, Efremov L, Zhang ZY, Thijs L, Wei FF, Huang QF, Luttun A, Verhamme P, Nawrot TS, et al.. ECG voltage in relation to peripheral and central ambulatory blood pressure.Am J Hypertens. 2018; 31:178–187. doi:10.1093/ ajh/hpx157
6. Huang QF, Aparicio LS, Thijs L, Wei FF, Melgarejo JD, Cheng YB, Sheng CS, Yang WY, Gilis-Malinowska N, Boggia J, et al.; IDCARS (International Database of Central Arterial Properties for Risk Stratification) Investigators. Cardiovascular endpoints and mortality are not closely associated with central than peripheral pulsatile blood pressure components. Hypertension. 2020; 76:350–358. doi: 10.1161/ HYPERTENSIONAHA.120.14787
7. Cheng HM, Chuang SY, Sung SH, Yu WC, Pearson A, Lakatta EG, Pan WH, Chen CH. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks.J Am Coll Cardiol. 2013; 62:1780–1787. doi: 10.1016/j.jacc. 2013.06.029
8. Booysen HL, Norton GR, Maseko MJ, Libhaber CD, Majane OH, Sareli P, Woodiwiss AJ. Aortic, but not brachial blood pressure category enhances the ability to identify target organ changes in normotensives. J Hypertens. 2013; 31:1124–1130. doi:10.1097/HJH. 0b013e 328360802a
9. Chuang SY, Chang HY, Cheng HM, Pan WH, Chen CH. Prevalence of hypertension is defined by central blood pressure measured using a Type II device in a nationally representative cohort.Am J Hypertens. 2018; 31:346 –354. doi:10.1093/ajh/ hpx178
10. Yu S, Xiong J, Lu Y, Chi C, Teliewubai J, Bai B, Ji H, Zhou Y, Fan X, Blacher J, et al.. The prevalence of central hypertension defined by a central blood pressure type I devise and its association with target organ damage in the community-dwelling elderly Chinese: The Northern Shanghai Study. J Am Soc Hypertens. 2018; 12: 211– 219. doi: 10.1016/ j.jash.2017.12 .013
11. Chuang SY, Chang HY, Cheng HM, Pan WH, Chen CH. Impacts of the New 2017 ACC/AHA hypertension guideline on the prevalence of brachial hypertension and its concordance with central hypertension. Am J Hypertens. 2019; 32:409–417. doi: 10.1093/ ajh/hpz008
12. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al.. 2017 ACC/AHA/AAPA/ ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018; 71:e127– e248. doi: 10.1161/HYP.00000 00000000080
13. Boggia J, Luzardo L, Lujambio I, Sottolano M, Robaina S, Thijs L, Olascoaga A, Noboa O, Struijker-Boudier HA, Safar ME, et al.. The diurnal profile of central hemodynamics in a general uruguayan population.Am J Hypertens. 2016; 29:737–746. doi: 10.1093/ajh/hpv169
14. Aparicio LS, Huang QF, Melgarejo JD, Wei DM, Thijs L, Wei FF, Gilis-Malinowska N, Sheng CS, Boggia J, Niiranen TJ, et al.; International Database of Central Arterial Properties for Risk Stratification (IDCARS) Investigators. The international database of central arterial properties for risk stratification: research objectives and baseline characteristics of participants. Am J Hypertens. 2022; 35:54–64. doi: 10.1093/ajh /hpab139
15. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Ass. 2013; 310:2191–2194. doi: 10.1001/ jama.2013.281053
16. Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform.Hypertension. 2001; 38:932–937. doi: 10.1161 /hy1001. 096106
17. World Health Organization. Global Status Report on Alcohol and Health 2018. World Health Organization, 2018.
18. Cheng YB, Thijs L, Zhang ZY, Kikuya M, Yang WY, Melgarejo JD, Boggia J, Wei FF, Hansen TW, Yu CG, et al.. Outcome-driven thresholds for ambulatory blood pressure based on the New American College of Cardiology/American Heart Association Classification of Hypertension. Hypertension. 2019; 74:776–783. doi: 10.1161/ HYPERTENSIONAHA.119.13512
19. Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution.Stat Med. 1997; 16:791–801. doi: 10. 1002/(sici) 1097-0258(1997 0415 ) 16:7<791::aid-sim500>3.0.co;2-#
20. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424. doi:10.1080/ 00273171.2011.56878 6
21. O’Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth.Vasc Med. 2000; 5:141–145. doi: 10.1177/ 1358836X0000500303
22. Palatini P, Rosei EA, Avolio A, Bilo G, Casiglia E, Ghiadoni L, Giannattasio C, Grassi G, Jelakovich B, Julius S, et al.. Isolated systolic hypertension in the young: a position paper endorsed by the European Society of Hypertension. J Hypertens. 2018; 36:1222–1236. doi: 10. 1097/HJH. 0000000000001726
23. Herbert A, Cruickshank JK, Laurent S, Boutouyrie P; Reference Values for Arterial Measurements Collaboration. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014; 35:3122–3133. doi: 10.1093/ eurheartj/ehu293
24. Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, Boutouyrie P, Chen CH, Chowienczyk P, Cockcroft JR, et al.. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization.Eur Heart J. 2017; 38:2805–2812. doi: 10.1093/eurheartj/ ehw632
25. Picone DS, Schultz MG, Otahal P, Aakhus S, Al-Jumaily AM, Black JA, Bos WJ, Chambers JB, Chen CH, Cheng HM, et al.. Accuracy of cuff-measured blood pressure: systematic reviews and meta-analyses. J Am Coll Cardiol. 2017; 70:572–586. doi: 10.1016/j.jacc. 2017.05 .064
26. Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: the importance of brachial-to-radial pressure amplification.Hypertension.2005;46:244–248. doi: 10.1161/01. HYP.0000166723.07809.7e
27. Odili AN, Chori BS, Danladi B, Yang WY, Zhang ZY, Thijs L, Wei FF, Nawrot TS, Kuznetsova T, Staessen JA. Electrocardiographic left ventricular hypertrophy in relation to peripheral and central blood pressure indices in a Nigerian population. Blood Press. 2020; 29:39–46. doi: 10.1080/ 08037051.2019.1646610
28. Boggia J, Thijs L, Hansen TW, Li Y, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, et al.; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes Investigators. Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57: 397–405. doi: 10.1161/ HYPERTENSIONAHA.110.156828
29. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFÉ) study. Circulation. 2006;113:1213– 1225. doi: 10.1161/ CIRCULATION AHA.105.595496
Credit: Cheng YB, Thijs L, Aparicio LS, Huang QF, Wei FF, Yu YL, Barochiner J, Sheng CS, Yang WY, Niiranen TJ, Boggia J, Zhang ZY, Stolarz-Skrzypek K, Gilis-Malinowska N, Tikhonoff V, Wojciechowska W, Casiglia E, Narkiewicz K, Filipovský J, Kawecka-Jaszcz K, Wang JG, Li Y, Staessen JA; International Database of Central Arterial Properties for Risk Stratification (IDCARS) Investigators. Risk Stratification by Cross-Classification of Central and Brachial Systolic Blood Pressure. Hypertension. 2022May;79(5):1101-1111. doi: 10.1161/HYPERTENSIONAHA. 121.18773. Epub 2022 Mar 4. PMID: 35240865; PMCID: PMC8997688.