Vinh-An D Vo a, Mazen K.Khalil a b, Majdi N.Al-Hasan a c
a. University of South Carolina School of Medicine, Columbia, SC, USA
b. Department of Internal Medicine, Division of Cardiology, Prisma Health Midlands, Columbia, SC, USA
c. Department of Internal Medicine, Division of Infectious Diseases, Prisma Health Midlands, Columbia, SC, USA
Abstract
Objectives
This retrospective cohort study examines incidence, risk factors, and clinical outcomes of acute myocardial infarction (AMI) and acute ischemic stroke (AIS) within one year of gram-negative bloodstream infection (GN-BSI) based on predefined clinical criteria.
Methods
Hospitalized adults with GN-BSI at Prisma Health-Midlands hospitals in South Carolina, USA from 2010 through 2015 were identified. Kaplan-Meier analysis was used to determine the incidence of AMI and AIS within one year after GN-BSI. Multivariate Cox proportional hazards regression models were used to examining risk factors for AMI or AIS and their impact on 1-year mortality.
Results
Among 1292 patients with GN-BSI, 263 and 17 developed AMI and AIS within 1-year with incidences of 23.4% and 1.9%, respectively. The majority of AMI were type 2 (164; 62%); 99 patients had type 1 AMI with an incidence of 8.9%. Age >65 years (hazard ratio [HR] 1.52, 95% CI: 1.17–1.99), prior coronary artery disease or stroke (HR 1.74, 95% CI: 1.34–2.25), hypertension (HR 1.55, 95% CI: 1.13–2.15), end-stage renal disease (HR 1.52, 95% CI: 1.09–2.08), and quick Pitt bacteremia score (HR 1.55 per point, 95% CI: 1.40–1.72) were predictors of AMI/AIS. Development of type 1 AMI or AIS after GN-BSI was independently associated with increased 1-year mortality (HR 1.47, 95% CI: 1.03–2.07).
Conclusions
AMI and AIS occur frequently within one year of GN-BSI and have a negative impact on 1-year survival. Future randomized clinical trials are needed to determine the most effective clinical interventions for the prevention of AMI/AIS following BSI in high-risk patients and improve survival after these events.
Keywords:
Coronary artery disease, Acute coronary syndrome, Cardiovascular disease, Cerebrovascular disease, Bacteremia, Sepsis
1. Introduction
Over half a million individuals develop bloodstream infection (BSI) every year in the United States 1. An increased risk of acute myocardial infarction (AMI) and acute ischemic stroke (AIS) has been reported after various acute infections, including pneumonia, sepsis, and BSI 2, 3, 4, 5, 6, 7, 8. A large population-based cohort study in Denmark estimated a combined 5% risk of AMI or AIS within one year of BSI, using administrative data based on International Classification of Diseases (ICD) codes 9. Patients with BSI had a significantly higher risk of AMI and AIS than both population and hospitalized controls 9. To our knowledge, the incidence of AMI and AIS after BSI has not been established based on clinical data in the U.S. population. This retrospective observational cohort study aims to determine the incidence and risk factors of AMI and AIS within one year of gram-negative BSI based on predefined criteria. The study also examines the impact of cardiovascular and cerebrovascular events on 1-year mortality.
2. Methods
2.1. Settings
The study was conducted at Prisma Health-Midlands hospitals in Columbia, South Carolina, USA. The healthcare system includes three hospitals in Columbia, Richland, Baptist and Parkridge hospitals. The three community hospitals combine for >1100 licensed beds and provide primary and speciality care for the local population. The Institutional Review Board at Prisma Health approved the study and waived informed consent.
2.2. Definitions
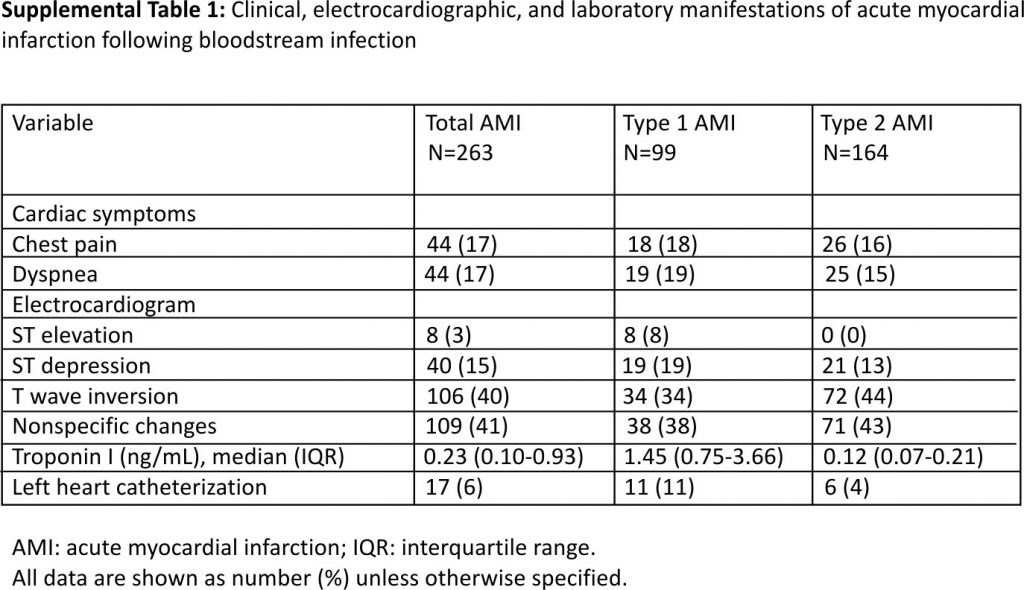
Gram-negative BSI was defined as monomicrobial growth of aerobic or facultative anaerobic gram-negative bacilli in blood cultures. Blood cultures were processed using standard microbiology techniques according to the Clinical and Laboratory Standards Institute. The primary source of BSI was defined according to the Centers for Disease Control and Prevention criteria 10. The quick Pitt bacteremia score was used to measure the acute severity of illness in patients with BSI 11,12. The fourth universal definition of myocardial infarction was used to classify AMI based on clinical, electrocardiographic (ECG), and laboratory criteria 13. Type 1 AMI involved myocardial injury and related ECG abnormalities and was further divided into ST-elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI). Type 2 AMI was related to the myocardial oxygen supply-demand mismatch. AIS was defined based on clinical and radiographic criteria 14.
2.3. Case ascertainment
In this retrospective cohort study, all patients ≥18 years old with first episodes of monomicrobial BSI due to gram-negative bacilli from January 1, 2010, to December 31, 2015, at Prisma Health-Midlands hospitals were identified through microbiology laboratory databases as described previously 15.
2.4. Statistical analysis
Kaplan-Meier analysis was used to estimate the incidence of AMI or AIS following BSI. Patients were followed from the time of collection of index blood culture up to one year or until the development of AMI, AIS or death. Patients who died or were lost to follow-up within one year of BSI were censored on the date of death or last health care visit, respectively. Kaplan-Meier analysis was also used to calculate the 30-day and 1-year incidence of type 1 AMI, type 2 AMI, and AIS separately.
Cox proportional hazards regression was used to determine risk factors for AMI or AIS within one year of BSI. Variables were included in the multivariate Cox model based on clinical relevance or statistical significance (p-value <0.05 in the univariable analysis) using backward selection.
The secondary aim of this study was to examine the impact of type 1 AMI and AIS on one-year mortality following BSI. In this analysis, patients with BSI were categorized into three groups: those who developed type 1 AMI or AIS, those with type 2 AMI, and those without cardiovascular or cerebrovascular events within one year of BSI. Multivariate Cox proportional hazards regression was used to examine predictors of one-year mortality following BSI. Patients were followed for up to one year from onset of BSI or until death; patients lost to follow up were censored.
Since not all patients with BSI had serum troponins measured, a subgroup analysis of patients who had at least one measurement of serum troponin I was performed to minimize the potential impact of selection bias on the results. Multivariate Cox proportional hazards regression was used to examining the association between the highest serum troponin I value as a continuous variable and one-year mortality following BSI.
JMP Pro (version 13.0, SAS Institute Inc, Cary, North Carolina, USA) was used for statistical analysis. The level of significance for statistical testing was defined as p < 0.05 (2-sided) unless otherwise specified.
3. Results
3.1. Incidence of AMI and AIS after BSI
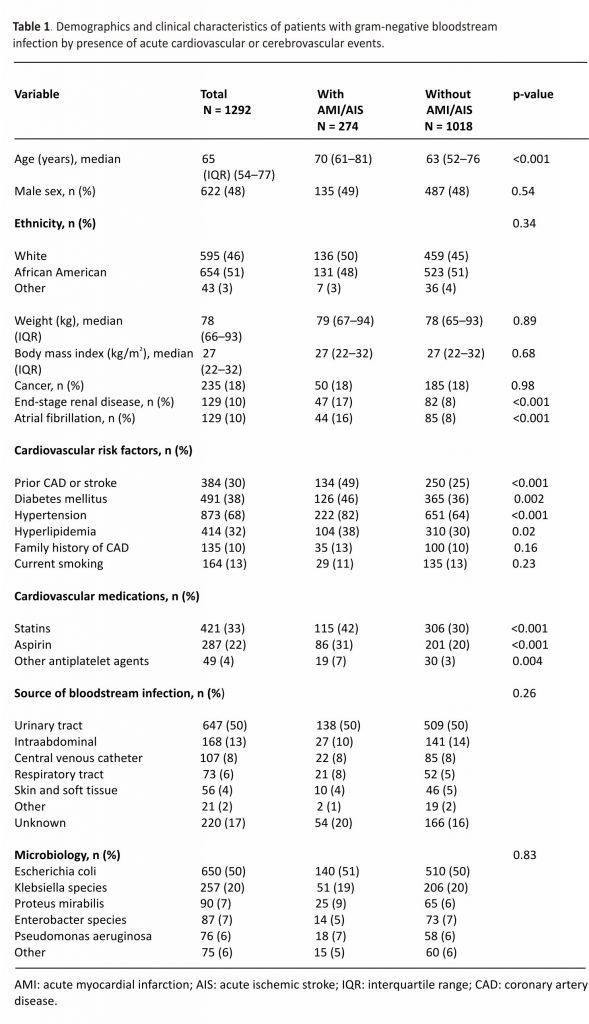
Among 1292 unique patients with gram-negative BSI, the median age was 65 years and 622 (48%) were men. Cardiovascular risk factors, namely, hypertension (68%), diabetes mellitus (38%), and hyperlipidemia (32%) were prevalent in this cohort. The urinary tract was the most common source of BSI; Escherichia coli was the most common bloodstream isolate (Table 1).
 Within one year of gram-negative BSI, 274 patients developed either AMI or AIS with an overall incidence of 24.5%. Most patients developed an AMI (263; 96.0%) with type 2 AMI being the most common (164; 62%). Of the 99 patients who developed type 1 AMI, 8 had STEMI and 91 had NSTEMI with a combined incidence of 8.9%. Only 35/263 (13%) patients with AMI had corresponding ICD codes for AMI during the index hospitalization(s), including 33/99 (33%) with type 1 and only 2/164 (1.2%) with type 2 AMI. The majority of AMI/AIS occurred within 30 days of gram-negative BSI (Table 2).
Within one year of gram-negative BSI, 274 patients developed either AMI or AIS with an overall incidence of 24.5%. Most patients developed an AMI (263; 96.0%) with type 2 AMI being the most common (164; 62%). Of the 99 patients who developed type 1 AMI, 8 had STEMI and 91 had NSTEMI with a combined incidence of 8.9%. Only 35/263 (13%) patients with AMI had corresponding ICD codes for AMI during the index hospitalization(s), including 33/99 (33%) with type 1 and only 2/164 (1.2%) with type 2 AMI. The majority of AMI/AIS occurred within 30 days of gram-negative BSI (Table 2).
3.2. The clinical course of AMI
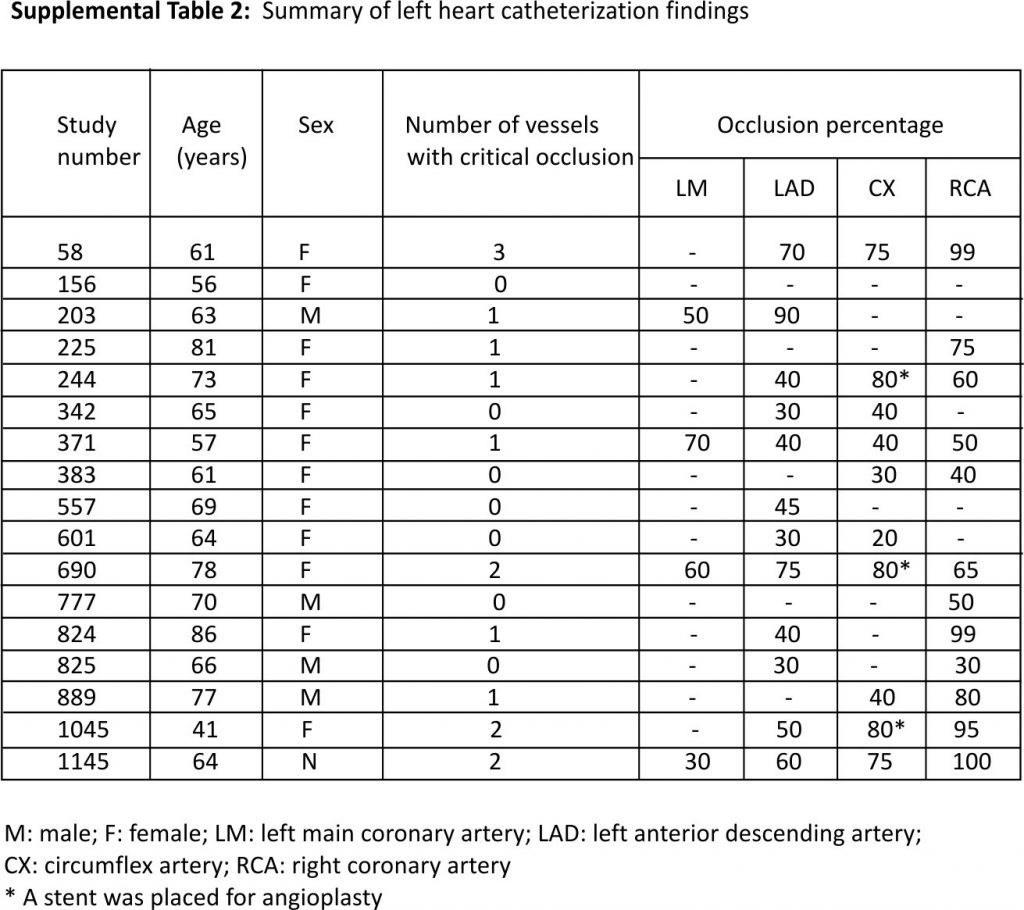
The median time from collection of index blood culture to the diagnosis of AMI was 0.67 days (range 0.02–357.5; interquartile range 0.22–11.78 days). Chest pain and dyspnea were the most common symptoms of AMI. ECG findings in patients with type 1 and type 2 AMI are summarized in Supplemental Table 1. The median serum troponin I was 1.45 ng/mL in type 1 AMI (range 0.40–112.5) and 0.12 ng/mL in type 2 AMI (range 0.04–1.0). Most patients with AMI received medical therapy; 17 patients (6.5%) underwent left heart catheterization. Of these 17 patients, 6 (35%) had no critical obstructive vascular disease, 7 (41%) had critical occlusion in one coronary artery, 3 (18%) had an obstruction in 2 arteries, and the remaining 1 (6%) had 3-vessel disease. The right coronary and circumflex arteries were the most commonly involved blood vessels (Supplemental Table 2). Three patients underwent stent placement for critical coronary stenoses.
3.3. Risk factors for AMI and AIS
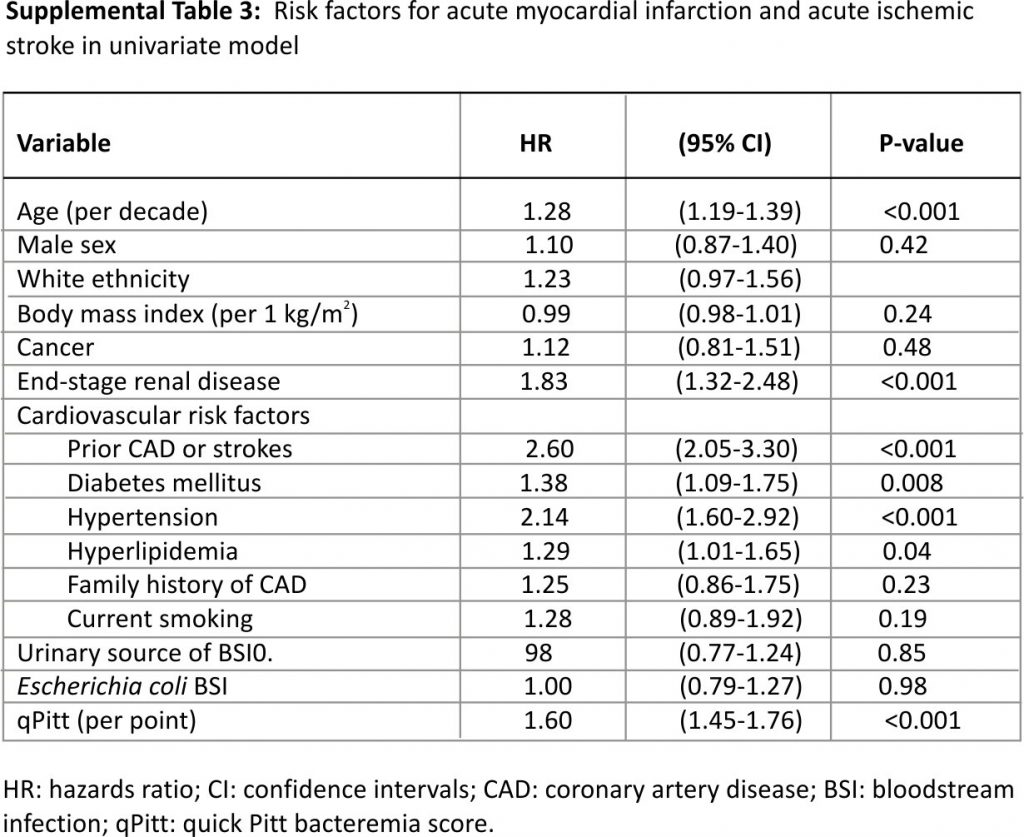
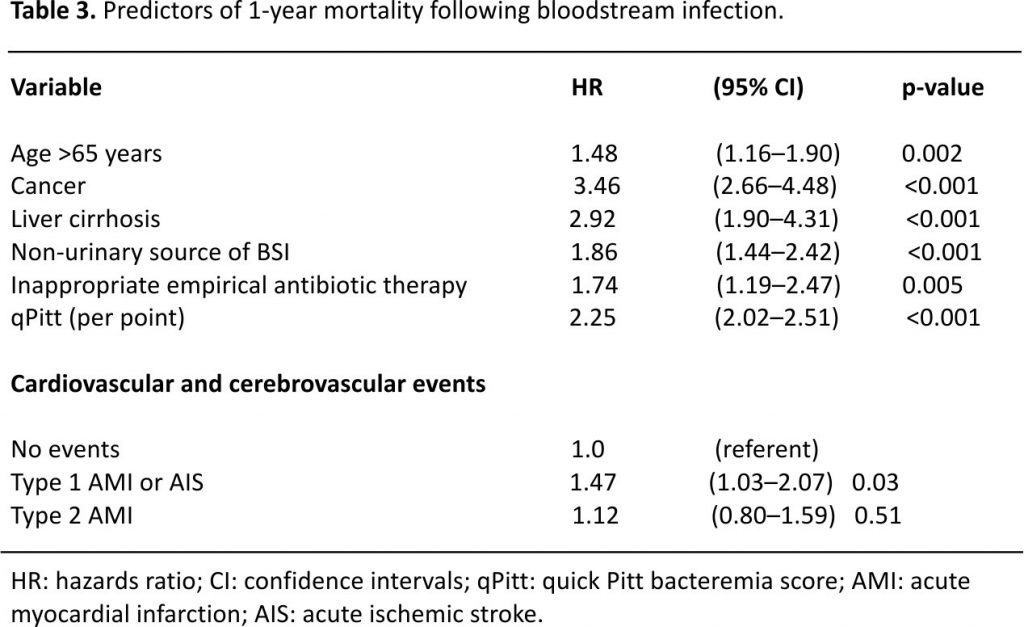
Univariate Cox model results for risk factors of AMI or AIS are demonstrated in Supplemental Table 3. The multivariate model identified five independent risk factors for AMI or AIS following BSI. Age >65 years (hazard ratio [HR] 1.52, 95% confidence intervals [CI]: 1.17–1.99), prior coronary artery disease (CAD) or stroke (HR 1.74, 95% CI: 1.34–2.25), hypertension (HR 1.55, 95% CI: 1.13–2.15), end-stage renal disease (HR 1.52, 95% CI: 1.09–2.08), and high acute severity of illness as measured by the quick Pitt bacteremia score (HR 1.55 per point, 95% CI: 1.40–1.72) were predictors for AMI/AIS. The incidence of AMI or AIS increased from 3.8% in patients without any risk factors to >50% in those with ≥4 risk factors (Fig. 1).
3.4. Impact of type 1 AMI and AIS on survival
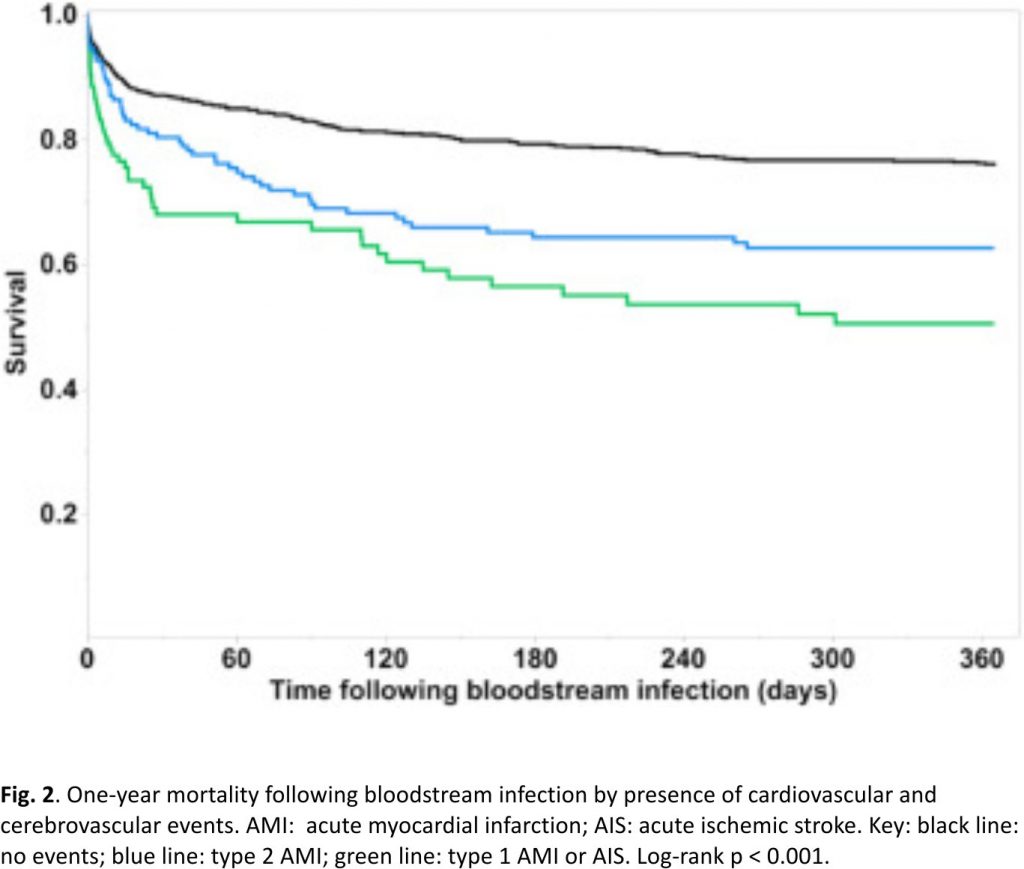
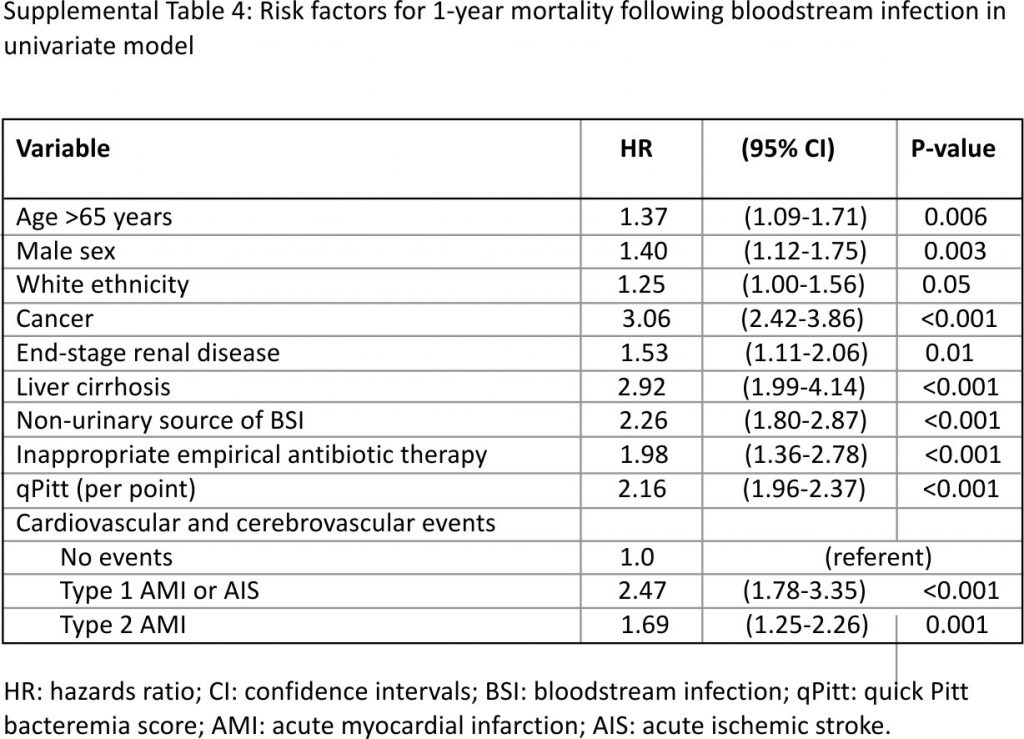
A total of 314 patients died within 1-year of BSI with an overall 1-year mortality of 28.1%. One-year mortality was 49.5% in those who developed type 1 AMI or AIS following BSI, 37.4% in patients with type 2 AMI, and 24.3% in the absence of these events (log-rank p < 0.001; Fig. 2). Univariate Cox model results for risk factors of 1-year mortality following BSI are shown in Supplemental Table 4. After adjustments in a multivariate model, development of type 1 AMI or AIS following BSI was independently associated with an increase in 1-year mortality (HR 1.47, 95% CI: 1.03–2.07; p = 0.03) as demonstrated in Table 3.
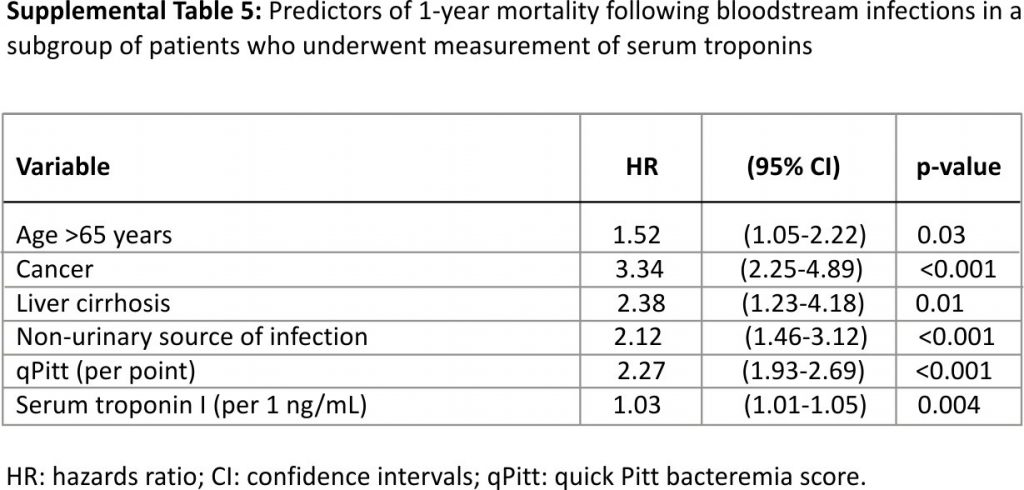
In a subgroup analysis of 492 patients who underwent at least one measurement of serum troponin I, 263 (53.5%) had elevated troponin I (≥0.04 ng/mL) and the remaining 229 had serum troponin I < 0.04 ng/mL. After adjustment for other variables in the multivariate Cox model, there was a 3% relative increase in 1-year mortality with each increase in troponin I of 1 ng/mL (Supplemental Table 5).
4. Discussion
4.1. Variable risk of AMI and AIS after BSI based on the methodology
In this large cohort, up to one-fourth of patients developed AMI or AIS within one year of gram-negative BSI. Although most events represented myocardial oxygen supply-demand mismatch (type 2 AMI), type 1 AMI and AIS occurred in 8.9% and 1.9% of patients, respectively. The study identified risk factors for AMI and AIS after BSI and determined the prognostic impact of these events on long-term survival.
To our knowledge, this is the first study to determine the incidence of AMI and AIS following BSI in the U.S. using predefined objective criteria. The incidence of AMI following BSI in this study was relatively high. It was about 5 times higher than previously estimated in the Danish population using administrative data 9. Although this may be due to dissimilarities between the two study populations, it is more likely due to differences in methodology. Using ICD codes in the Danish study likely underestimated the incidence of AMI 9. In the current study, only 1 in 7 patients with objective criteria for AMI had corresponding ICD codes during the index hospitalization(s), including one-third of those with type 1 AMI. This is conceivable since most type 1 and type 2 AMI in the current study occurred in the absence of typical cardiac symptoms such as chest pain or dyspnea. In addition, the number of patients who underwent coronary angiography (which usually adds an AMI ICD code) was low likely due to the high acute severity of illness. High clinical suspicion for cardiovascular and cerebrovascular events in patients who have nonspecific symptoms or who are feeling unwell despite appropriate management of BSI prompted cardiac or stroke work up in this population. Similar to the results of the study in Denmark, the first 30 days after Gram-negative BSI represented the highest risk of AMI.
4.2. Prevention of AMI and AIS after BSI in high-risk patients
Risk factors for AMI or AIS following BSI in this cohort included well-established cardiovascular and cerebrovascular risk factors in the general population (older age, prior CAD or stroke, and hypertension) 16. The current study identified the end-stage renal disease and high acute severity of illness as two additional risk factors in patients with BSI. The absence of diabetes mellitus as an independent risk factor was notable in the current study. This was likely due to sampling size and considerable overlap between diabetes mellitus, hypertension, and end-stage renal disease that might have limited the power of the multivariate model to identify all risk factors for AMI/AIS. High acute severity of illness is a surrogate for the stress imposed on the heart in the setting of acute infections. Most cardiovascular events occurred within 30 days of BSI. Acute inflammation in the setting of BSI is likely another contributing factor to AMI and AIS, particularly in late thrombotic events 17. It would be useful to examine the association between these events and an objective inflammatory marker such as C-reactive protein in future studies.
The high incidence of AMI or AIS following BSI prompts future randomized clinical trials for the prevention of these events. The use of statins, aspirin, and other antiplatelet agents has been suggested for the prevention of AMI and AIS after serious infections 18. The ability to estimate the incidence of AMI or AIS based on the number of risk factors in the current study assists in designing future randomized clinical trials for the prevention of these events in high-risk patients with BSI. Enrollment of patients with ≥2 risk factors seems reasonable since the incidence of AMI or AIS exceeds 20% in that setting. In addition, three-quarters of AMI and AIS in the current study occurred within 12 days of gram-negative BSI. This was consistent with the results of a previous study in Denmark that demonstrated the highest risk of cardiovascular and cerebrovascular events within 30 days of BSI 9. This relatively short time frame implies that any future interventions for the prevention of AMI and AIS should start soon after hospitalization for BSI.
4.3. Modification of long-term outcomes after type 1 AMI and AIS
Development of type 1 AMI or AIS after BSI had a negative impact on one-year survival. This was consistent with the results of a recent population-based cohort study in Queensland, Australia that demonstrated an increase in 1-year mortality in patients who developed AMI after Staphylococcus aureus BSI than those without AMI 19. This highlights the importance of high suspicion for AMI and AIS following BSI that allows early recognition, prompt diagnosis, and appropriate management. Identification of serum troponin I level as a prognostic marker in patients with BSI is a novel finding of the current study. These results improve our understanding of the impact of cardiovascular events on the long-term survival of patients with BSI, sepsis, and other serious infections. This also opens the horizon for future investigations to identify the most appropriate short- and long-term management plans to improve the outcomes of these patients. Optimal medical management with beta-blockers, statins, antiplatelet agents, and anticoagulants is recommended in patients with AMI 20. However, the dose and timing of initiation of these agents remain challenging in this setting. Many patients with BSI, particularly early in the course of illness, may not tolerate beta-blockers due to relative hemodynamic instability. Similarly, administration of antiplatelet agents and anticoagulation therapy may not be possible in critically -ill patients with suspicion of disseminated intravascular coagulation or in those requiring surgical procedures to control the source of BSI. The role of anti-inflammatory agents such as low-dose colchicine remains to be determined in these patients 21. Optimization of medical management and modification of cardiovascular risk factors after discharge from the hospital is also crucial to reduce the long-term impacts of these events. This presents opportunities for improvement of the transitions of care from the hospital to primary care or subspecialty clinics.
The role of invasive management of AMI after BSI remains unclear. The patients included in the study had severe infections; some patients died within days of admission. In addition, some patients developed multi-organ failure which makes them less than ideal candidates for invasive management. This in part explains the fact that only a few patients in the current study underwent left heart catheterization after AMI. One-third of those who underwent invasive management did not have hemodynamically significant coronary lesions. Since the majority of AMI were likely due to myocardial oxygen supply-demand mismatch in the setting of an acute infection, it remains possible that many patients may have undiagnosed severe CAD given the high prevalence of cardiovascular risk factors in this population.
4.4. Strengths and limitations
Definition of AMI and AIS based on objective criteria rather than ICD codes represents a unique approach and adds strength to the current study. Estimation of the incidence of cardiovascular and cerebrovascular events by the number of risk factors provides a practical tool for designing future randomized clinical trials. The study has some limitations. The observational design may contribute to missing some events, particularly in patients with nonspecific symptoms who may not have complete cardiac or neurologic workup for diagnosis of AMI or AIS. In addition, retrospective chart review may underestimate the prevalence of poorly documented risk factors in existing medical records such as smoking status and family history of CAD. The study included only gram-negative BSI rather than all BSI. However, a previous large population-based study demonstrated a comparable incidence of cardiovascular and cerebrovascular events after gram-negative and gram-positive BSI 9. Finally, the study lacked a control group of patients without gram-negative BSI to compare the risk of AMI or AIS between the two populations. However, the one-year risk of AMI/AIS following gram-negative BSI in the current study was substantially higher than hospital and population-based controls with relatively comparable age in a previous study in Denmark (4.1% and 1.8%, respectively) 9.
5. Conclusions
AMI and AIS occurred in up to one-quarter of patients in the first year following gram-negative BSI. Most cardiovascular and cerebrovascular events occurred early in the course of acute illness with three-quarters diagnosed within 12 days of BSI. The majority of these events represented myocardial oxygen supply-demand mismatch. However, more than 10% of patients had either type 1 AMI or AIS within one year of BSI. The study results improve the understanding of the intricate relationship between acute infection, inflammation, and vascular disease. Identification of risk factors for AMI and AIS after BSI and estimation of the incidence by the number of risk factors assist in designing future randomized clinical trials to determine the most effective clinical interventions for the prevention of cardiovascular and cerebrovascular ischemic complications. The significant increase in 1-year mortality in patients who develop type 1 AMI or AIS after BSI encourage prompt recognition, appropriate workup, and optimal inpatient and ambulatory management of these events to improve long-term survival.
Author contributions
Vinh-An D. Vo: Data curation; Funding acquisition; Investigation; Methodology; Visualization; Writing – original draft.
Mazen K. Khalil: Data curation; Investigation; Methodology; Resources; Supervision; Validation; Visualization; Writing – review & editing.
Majdi N. Al-Hasan: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft.
Funding information
VDV received funding from the Student Opportunities for Academic Research program at the University of South Carolina School of Medicine Research Center for Transforming Health and the American Heart Association. The study had no other sources of funding.
Transparency declarations and potential conflicts of interest
VDV, MKK, and MNA: no conflicts.
VDV, MKK and MNA have full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the analysis.
Acknowledgements
The authors thank Prisma Health-Midlands Antimicrobial Stewardship and Support Team in South Carolina, the USA for their help in facilitating the conduct of this study.
The preliminary results of this study were presented in part virtually at the American College of Cardiology annual meeting on March 28–30, 2020.
Appendix A. Supplementary data
References
[1] M. Goto, M.N. Al-Hasan. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect., 19 (2013), pp. 501-509
[2] R.L. Levine, J.R. LeClerc, J.E. Bailey, M.J. Monberg, S. Sarwat. Venous and arterial thromboembolism in severe sepsis Thromb. Haemost., 99 (2008), pp. 892-898
[3] V.F. Corrales-Medina, D.M. Musher, G.A. Wells, J.A. Chirinos, L. Chen, M.J. Fine. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and associations with short term mortality. Circulation, 125 (2012), pp. 773-781
[4] V.F. Corrales-Medina, J. Serpa, A.M. Rueda, et al. Acute bacterial pneumonia is associated with the occurrence of acute coronary syndromes. Medicine (Baltim.), 88 (2009), pp. 154-159
[5] D.M. Musher, A.M. Rueda, A.S. Kaka, S.M. Mapara. The association between pneumococcal pneumonia and acute cardiac events. Clin. Infect. Dis., 45 (2007), pp. 158-165
[6] T.W. Perry, M.J. Pugh, G.W. Waterer, et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am. J. Med., 124 (2011), pp. 244-251
[7] A.J. Walkey, R.S. Wiener, J.M. Ghobrial, L.H. Curtis, E.J. Benjamin. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. J. Am. Med. Assoc., 306 (2011), pp. 2248-2254
[8] E.S. Schut, M.J. Lucas, M.C. Brouwer, M.D. Vergouwen, A. van der Ende, D. van de Beek. Cerebral infarction in adults with bacterial meningitis Neurocrit. Care, 16 (2012), pp. 421-427
[9] M. Dalager-Pedersen, M. Søgaard, H.C. Schønheyder, H. Nielsen, R.W. Thomsen. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study Circulation, 129 (2014), pp. 1387-1396.
[10] T.C. Horan, M. Andrus, M.A. Dudeck CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting Am. J. Infect. Contr., 36 (2008), pp. 309-332
[11] S.E. Battle, M.R. Augustine, C.M. Watson, P.B. Bookstaver, J. Kohn, W. Owens, L.M. Baddour, M.N. Al-Hasan. Derivation of a quick Pitt bacteremia score to predict mortality in patients with gram-negative bloodstream infection Infection, 47 (2019), pp. 571-578
[12]M.N. Al-Hasan, L.M. Baddour. The resilience of the Pitt bacteremia score: three decades and counting Clin. Infect. Dis., 70 (2019), pp. 1834-1836
[13] K. Thygesen, J.S. Alpert, A.S. Jaffe, et al. Fourth universal definition of myocardial infarction (2018) Circulation, 138 (2018), pp. e618-e651
[14] R.L. Sacco, S.E. Kasner, J.P. Broderick, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association /American Stroke Association Stroke, 44 (2013), pp. 2064-2089
[15] P.B. Bookstaver, E.B. Nimmich, T.J. Smith 3rd, J.A. Justo, J. Kohn, K.L. Hammer, et al. Cumulative effect of antimicrobial stewardship and rapid diagnostic testing bundle on early streamlining of antimicrobial therapy in gram-negative bloodstream infections. Antimicrob. Agents Chemother., 61 (2017) e00189-17
[16] D.K. Arnett, R.S. Blumenthal, M.A. Albert, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines J. Am. Coll. Cardiol., 74 (2019), pp. 1376-1414 2019
[17] S. Yende, J.A. Kellum, V.B. Talisa, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open, 2 (2019), Article e198686
[18] A.J. Walkey. Preventing cardiovascular complications of acute infection: a missed opportunity? Circulation, 129 (2014), pp. 1375-1377
[19] J.F. McNamara, P.N.A. Harris, M.D. Chatfield, D.L. Paterson. Acute myocardial infarction and community-acquired Staphylococcus aureus bloodstream infection: an observational cohort study Clin. Infect. Dis. (2020), 10.1093/cid/ciaa1197 [Published online ahead of print]
[20] E.A. Amsterdam, N.K. Wenger, R.G. Brindis, et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol., 64 (2014), pp. e139-e228 2014
[21] J.C. Tardif, S. Kouz, D.D. Waters, et al. Efficacy and safety of low-dose colchicine after myocardial infarction N. Engl. J. Med., 381 (2019), pp. 2497-2505
Credits: Vo VD, Khalil MK, Al-Hasan MN. Risk and clinical outcomes of acute myocardial infarction and acute ischemic stroke following gram-negative bloodstream infection. Int J Cardiol Hypertens. 2021 Jan 23;8:100079. doi: 0.1016/j.ijchy.2021.100079. PMID: 33598654; PMCID: PMC7868809.