Vincenzo Savarino,1 Elisa Marabotto,1 Patrizia Zentilin,1 Maria Giulia Demarzo,1 Nicola de Bortoli,2 Edoardo Savarino3
1Department of Internal Medicine (DIMI), University of Genoa, Genoa, Italy; 2Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; 3Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy
Correspondence: Vincenzo Savarino, Department of Internal Medicine (DIMI), University of Genoa, Genoa, Italy. Email vsavarin@unige.it
Abstract:
Gastro-esophageal reflux disease (GERD) is a highly prevalent, chronic disorder, whose knowledge remains limited and the management of these patients changes continuously. This review provides a summary of the most recent advancements in the pathogenesis of this disease and the new drugs introduced into the market to overcome some of the unmet needs of traditional therapies. Nowadays, the most fruitful diagnostic examinations are 24-hour impedance-pH monitoring, which allows us to separate true NERD from esophageal functional disorders and high-resolution manometry, which helps to exclude the existence of motility disorders sharing the same symptoms of GERD. Proton pump inhibitors (PPIs) remain the first-choice therapy in the treatment of GERD, but a consistent proportion of these patients continue to experience symptoms despite their intake. These cases pertain mainly to the subpopulation with non-erosive reflux disease (NERD) and represent very challenging clinical situations, because it is mandatory to understand the reasons for PPI failure. The management of these difficult patients requires necessarily to test them and avoid the use of empiric treatments that are often unsuccessful, costly and potentially dangerous. Recently, several new drugs have been used to increase the defensive properties of this mucosa with promising results in randomized clinical trials.
Keywords: medical management of GERD, proton pump inhibitors, potassium competitive acid blockers, PPI-refractory patients, esophageal mucosal resistance, mucosal protective agents, bile acid sequestrant drug
Introduction
Gastroesophageal reflux disease (GERD) is defined as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications”.1 Typical esophageal symptoms include heartburn and regurgitation and, more rarely, chest pain and dysphagia. Extra-esophageal symptoms or signs with an established association with GERD on the basis of population-based studies are chronic coughs, hoarseness, asthma and dental erosions. However, these symptoms have potential etiologies other than GERD and, in the absence of concomitant typical GERD symptoms, the causal role of reflux remains difficult to prove. GERD complications are mainly represented by mucosal injury, the most common being reflux esophagitis, strictures, Barrett’s oesophagus and adenocarcinoma.
The prevalence of GERD is high in Western countries and ranges from 13% to 20% in the USA and from 9.8% to 18% in Europe, while it is lower in Asia (2.5–4.8%).2 Obesity, increasing age, a family history of reflux disease and chronic consumption of certain drugs (nitrates, calcium antagonists, benzodiazepines, etc.) are significant risk factors.3–5
GERD is a chronic disease with phases of recurrence and remission over time, but it can be considered a benign disorder from a prognostic point of view.6
The aim of this review is to provide a summary of the most recent studies on the unmet needs and the pharmacological management of GERD with particular attention to the new molecules that have enriched our therapeutic armamentarium. A computerized (PubMed databases) literature research was performed with a focus on the last five years (2016–2020). We used the following terms: “GERD”, “GORD”, “gastroesophageal reflux disease treatment or therapy” “functional heartburn”, “reflux hypersensitivity”, “PPI-refractory GERD”, “PPI failure”, “impedance-pH monitoring”, “esophageal pH-metry”, “Bravo system”, “high-resolution manometry”, “proton pump inhibitors”, “PPIs”, “H2 antagonists”, “potassium competitive acid blockers”, “P-CABs”, “GABAb agonists, anti-depressants”, “pain modulators”, “esophageal mucosal integrity”, “esophageal mucosal resistance”, “esophageal mucosal protective drugs”, “alginates”, “hyaluronic acid”, “esophageal medical devices”, “add-on therapy to PPIs”. We critically reviewed all full-text papers, including clinical trials, systematic reviews and meta-analyses, published in the English language. The number of clinical trials we considered in this review was 19 and that of systematic reviews was 14.
Pathophysiology of GERD
GERD is due to multiple mechanisms and motor alterations predominate, as shown in Figure 1. The anti-reflux barrier is thought to consist of the intrinsic pressure of the lower esophageal sphincter (LES), the extrinsic compression of the LES by the crural diaphragm and the acute angle of His. There are three prevalent mechanisms of reflux: transient LES relaxations (TLESRs), LES hypotension and anatomical disruption of the esophagogastric junction (EGJ), that is hiatus hernia.7
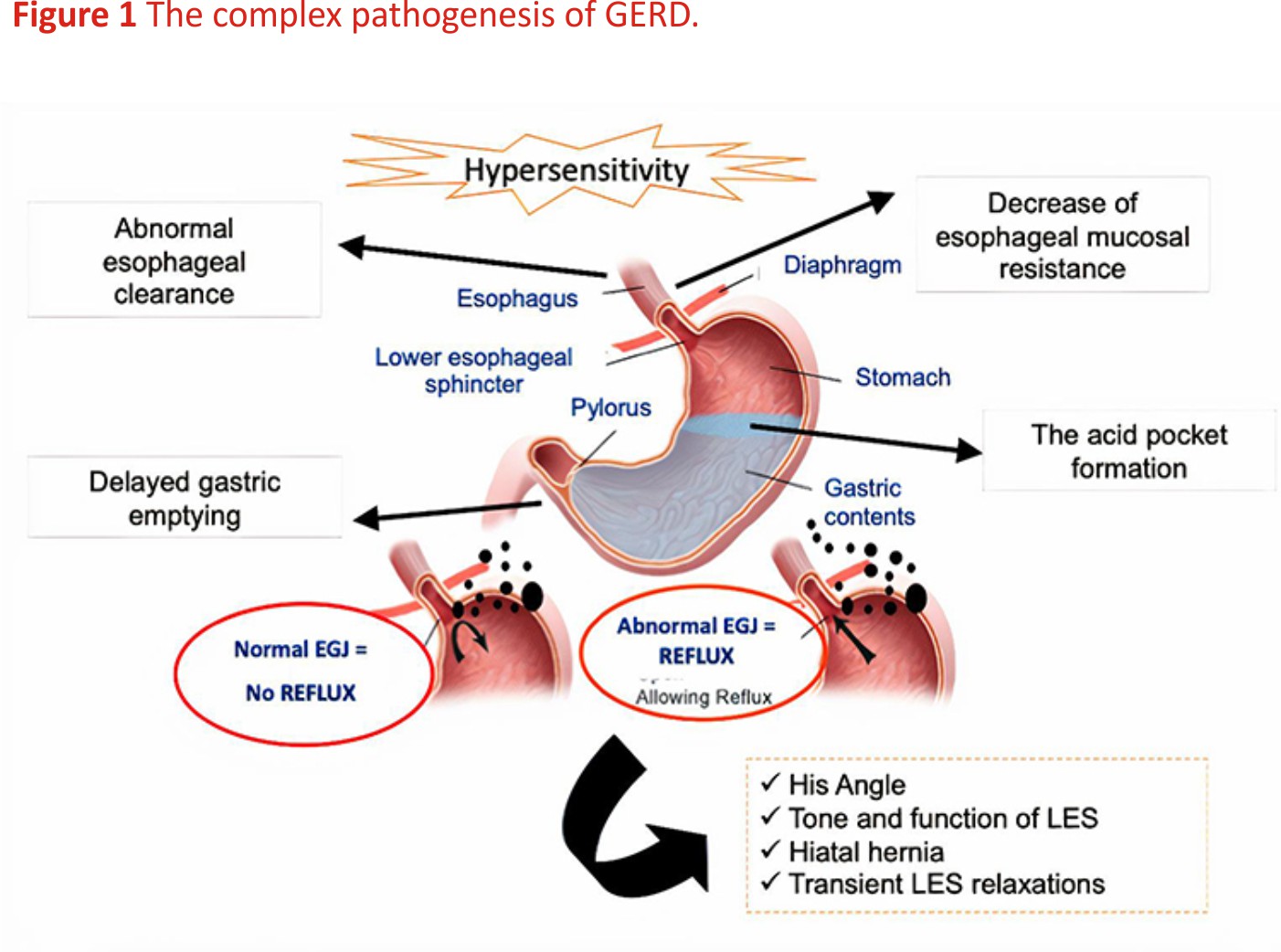 TLESRs represent the most important mechanism of reflux in healthy subjects and in a very large part of GERD patients; they occur during swallows to allow the passage of a bolus from the oesophagus into the stomach and are also induced by secondary peristalsis, which starts from the mid oesophagus as the effect of a vago-vagal reflex commencing with activation of gastric receptors primarily placed in the sub-cardiac region. Therefore, the primary stimulus which triggers a TLESR is gastric distension, often due to gastric air or the presence of a meal and this explains why TLESRs are mainly a postprandial phenomenon.8
TLESRs represent the most important mechanism of reflux in healthy subjects and in a very large part of GERD patients; they occur during swallows to allow the passage of a bolus from the oesophagus into the stomach and are also induced by secondary peristalsis, which starts from the mid oesophagus as the effect of a vago-vagal reflex commencing with activation of gastric receptors primarily placed in the sub-cardiac region. Therefore, the primary stimulus which triggers a TLESR is gastric distension, often due to gastric air or the presence of a meal and this explains why TLESRs are mainly a postprandial phenomenon.8
A second mechanism is LES hypotension and episodes of free reflux are observed only when the LES pressure is lower than 5 mmHg measured by manometry. This mechanism is particularly frequent in patients with scleroderma.9
A third factor, the presence of hiatal hernia favours gastroesophageal reflux by increasing the severity of oesophagal acid exposure. It alters the position of LES with respect to the crural diaphragm because these two important factors of the anti-reflux barrier are no longer coupled.10
In addition to the above mechanisms, impaired oesophagal clearance of refluxate may prolong the contact time with the mucosa of the distal part of the oesophagus and this contributes to generating symptoms or damaging the epithelium. Primary (swallow-induced) and secondary (distension-induced) peristalses are the main clearance events to clear the refluxate. Oesophagal motility disorders occur in about 30% of patients with GERD, with ineffective oesophagal motility being the most prevalent alteration.11 Although less relevant, also chemical clearance due to bicarbonates contained in the saliva is decreased in GERD.12 Recently, the relevance of oesophagal clearance has been shown even in neonates, where it enables the prediction of the response to PPI therapy. In a study by Nobile et al 13, a more efficient and rapid oesophagal clearance assessed by impedance-pH monitoring was significantly associated with a positive clinical response to anti-reflux therapy with omeprazole among newborns with increased acid oesophagal exposure time (AET).
Finally, delayed gastric emptying has been demonstrated in about 40% of GERD patients and this can favour the backflow of the material retained in the stomach.14
Although GERD treatment is mainly based at present on the use of antisecretory drugs to reduce acid reflux, there is no evidence of gastric acid hypersecretion in these patients.15 In fact, acid remains the most aggressive factor in determining mucosal damage or reflux symptoms. Chronic smoking is also considered a risk factor for GERD development.
In summary, the alteration of the anti-reflux barrier is sometimes associated with an inadequate clearing of refluxate and a delayed gastric emptying; in addition, dietetic factors, drugs able to reduce competence of LES and obesity contribute to induce the reflux of too much acid in the wrong place, that is the oesophagus, the mucosa of which is not familiar with this aggressive element.
In recent years, another mechanism, such as the reduction of defensive properties of oesophagal mucosa has been advocated in the pathogenesis of GERD.16
There are many studies showing that the mucosal resistance of the oesophagus is impaired in most patients with GERD, particularly in those with non-erosive reflux disease (NERD), who do not present mucosal lesions at endoscopy.16,17 Indeed, the presence of dilated intercellular spaces (DIS) is common in patients with true NERD and reflux hypersensitivity (RH) because of the presence of an impaired mucosal barrier.18 In patients with NERD it has been shown that microscopic esophagitis, including DIS, is significantly lower in controls (15%) and in patients with functional heartburn (FH) (13%) than in patients with RH (65%) and in those with an excess of acid (77%).19
Phenotypic Presentation of GERD
At the end of the second-millennium erosive esophagitis (EE) was identified with GERD, but in the last two decades, we have realized that patients with esophagitis represent only a minority (25–30%) of the entire spectrum of this disease, because about 70–75% of them pertain to the NERD phenotype, that is patients with typical reflux symptoms and devoid of any oesophagal lesion visible at endoscopy.20 Two large population-based epidemiological studies have demonstrated that the rate of endoscopy-negative cases can be as high as 75%.21,22
Current pathophysiological studies performed with the modern 24-hour impedance-pH monitoring have demonstrated that the NERD population is greatly heterogeneous from a pathophysiological point of view and can be subdivided into several subgroups23: about 40% of them have an excess of acid in their oesophagus (true NERD), while the remaining part (60%) have normal oesophagal acid exposure. This latter population can be further distinguished in the following subgroups: 1) patients with RH to both acid and weakly acidic reflux (about 40%) and patients with FH (about 20%). Patients with RH have a positive association between symptoms and episodes of reflux, while those with FH do not have any relationship with reflux events. According to Rome IV criteria for functional oesophagal disorders,24 patients with RH and FH are no more considered in the GERD population, but this has been questioned.25,26
Pharmacological Treatment of GERD
The multiple factors implicated in the pathogenesis of GERD and the various forms of clinical presentation of this disease do not permit us to manage our patients in the same way and to use a unique pharmacological treatment, which can be of benefit in each situation we may face within our routine daily practice. Therefore, the most practical therapeutic approach relies on targeting the individual elements of GERD pathophysiology according to each clinical situation and implies that our medical treatment will be always palliative because it is unable to control the functional alterations representing the real mechanisms of the disease. In this review we will list the most frequently used drugs to ameliorate the single or combined mechanisms of reflux, taking into consideration their chance of success in the different clinical presentations of GERD.
Acid Control
Proton Pump Inhibitors
Although gastric acid secretion is not increased in patients with GERD and the main pathophysiological alterations of this disease are represented by motility dysfunctions, the contact of acid with oesophagal mucosa remains a key factor in the generation of symptoms and in the induction of inflammatory lesions at the distal part of the oesophagus.27 Therefore, it is not surprising that the dominant medical treatment of patients with GERD focuses on inhibiting acid secretion.
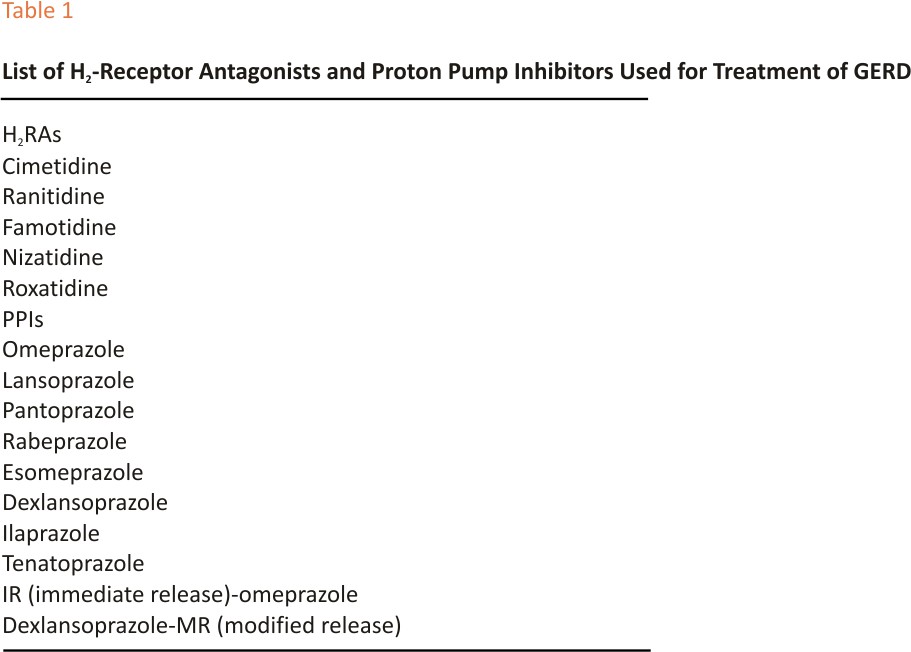
Proton pump inhibitors (PPIs) are the most powerful antisecretory drugs available because they bind to the H+K+ ATPase, which is the final step in the production of acid by the gastric parietal cell and therefore they have rapidly replaced the less potent H2-receptor antagonists (H2RAs) as first-choice drugs in the control of this relevant element in the pathogenesis of GERD.28 Table 1 shows a list of H2-RAs and PPIs used for the treatment of GERD. Despite the greater antisecretory activity of PPIs compared to H2RAs, their efficacy is variable, depending on the degree to which the clinical manifestations of the disease are more or less attributable to acid.
There is no doubt that the most responsive form of GERD is EE, particularly when it presents with the more severe degrees of mucosal damage, ranging from B and D of the well-known Los Angeles classification.29 There are many randomized clinical trials, systematic reviews and meta-analyses showing that PPIs are able to achieve a very high healing rate, proximal to 80–90% in these patients within 8–12 weeks of treatment. 30,31 This effectiveness in healing oesophagal mucosal alterations is associated with a quick resolution of typical symptoms of the disease, particularly heartburn when PPIs are compared with placebo and H2RAs. However, their optimal control of symptoms is reduced in the case of regurgitation, which can continue to persist despite these powerful antisecretory drugs.32 The efficacy of PPIs has been shown to be high also in patients with reflux-induced chest pain, as shown in a recent meta-analysis, in which six randomized controlled trials on patients studied with 24-hour oesophagal pH-metry, found a benefit in 56–85% of GERD positive patients compared with 0–17% of GERD negative ones.33 Also, dysphagia, which occurs in about one-third of patients with EE without strictures or cancer, resulted in to resolve in 83% of cases using PPI therapy.34
The excellent results of PPIs in EE are lower by a factor of 20%-30% in patients with the non-erosive form of GERD35 and this is due to the reduced pathogenetic role of acid in this population.36 In fact, we have already mentioned that patients with NERD are greatly heterogeneous from a pathophysiological point of view and acid is not responsible for heartburn in cases of RH to weakly acidic reflux and FH. These categories do not respond to antisecretory drugs because their symptoms are generated by factors other than increased or normal oesophagal acid exposure.
However, NERD patients with excess of acid in their oesophagus show the same benefit as the EE ones by PPI therapy.37
As GERD is a chronic condition with more or less frequent clinical relapse, maintenance therapy is needed for continued symptom control and esophagitis remission. Independently of the modality of maintenance therapy, on-demand or continuous, PPIs have been superior to H2RAs, prokinetics and placebo in preventing relapses of symptoms and oesophagal lesions over periods of times of one or more years.38,39
As to the management of extra-oesophagal symptoms of GERD (chronic cough, hoarseness, asthma), this aspect is highly controversial because the diagnosis of atypical GERD is very difficult and the risk of other etiologies sustaining these symptoms remains possible. Anyway, PPI therapy can be successful also in these patients, particularly when typical symptoms are concomitant, and therefore support the existence of gastroesophageal reflux as the cause of them.40
The PPI safety has been questioned in last years by the publication of many studies, mainly observational, on the occurrence of multiple adverse events,41 including hypomagnesemia, enteric infections, ischemic heart disease, kidney injury, pneumonia, dementia and nutritional deficiencies. However, this alarmism has been rejected in great part in recent reviews,42,43 due to the important methodological flaws of observational and retrospective studies performed to show the above adverse reactions and the lack of biological plausibility in the majority of risks reported in published papers.
PPI-Refractory GERD
Many studies have reported that a great number of patients with GERD symptoms, particularly heartburn, ranging from 19% to 44%, report either partial or complete lack of response to a standard PPI dose.44,45 In addition to incorrect dosage or timing of PPI intake, various mechanisms may induce this phenomenon, such as weakly acidic or bile reflux, mutations of hepatic cytochrome P-450, oesophagal hypersensitivity to physiological reflux, the presence of FH, which does not pertain anymore to the GERD realm.46 The main population responsible for PPI refractoriness is represented by NERD patients, who include both RH and FH, that is two clinical conditions in which the pathogenetic role of acid is reduced or absent.47
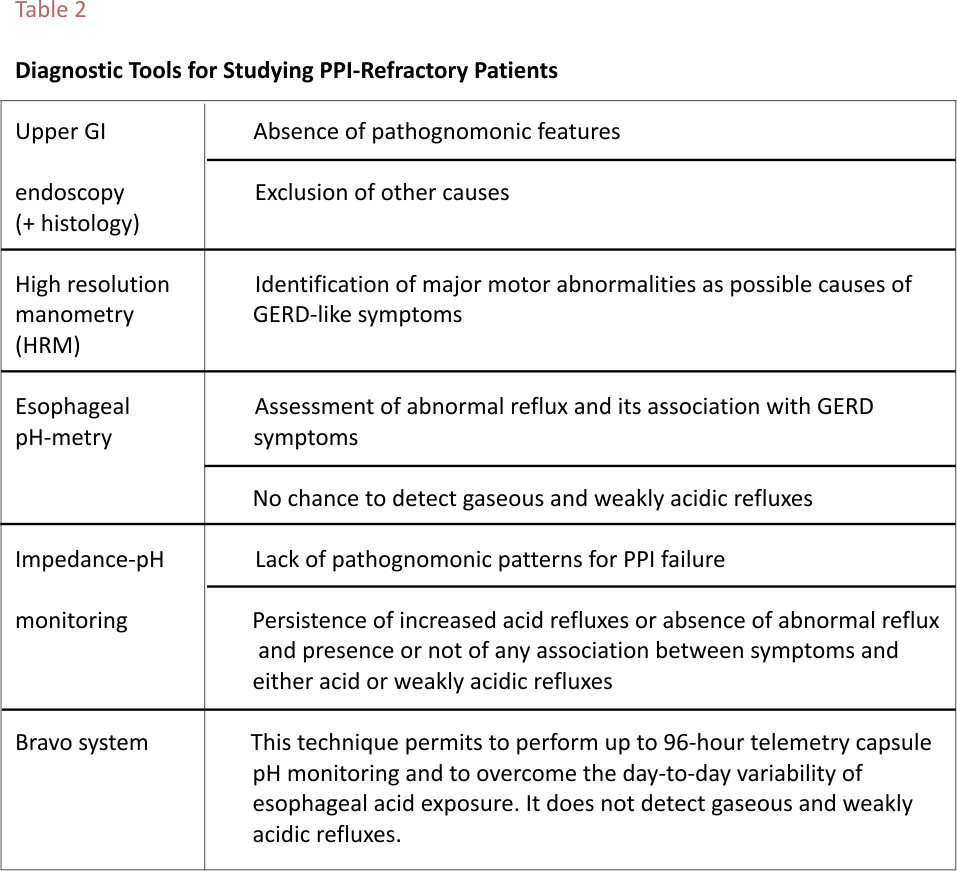
The management of non-responsive GERD patients is a challenging task in routine clinical practice and requires the use of objective diagnostic tools (Table 2) and among them, the most useful appear to be the prolonged registration of acid exposure time (AET) up to 96 hours by the Bravo system or 24-hour impedance-pH monitoring, in order to detect the real cause of PPI failure.48,49 In particular, FH has been found to be associated with both functional dyspepsia and irritable bowel syndrome, thus suggesting that these three conditions might share the same pathogenetic mechanism, that is increased visceral perception.46,50
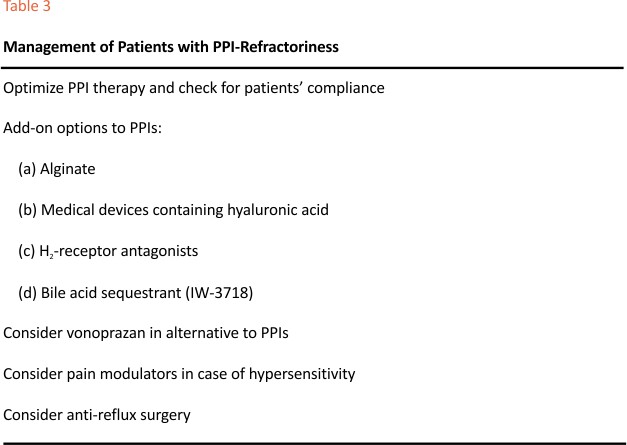
The therapeutic approach to these patients is complex and several options are available, although they are not sustained by a large body of randomized clinical trials. Table 3 shows the list of major treatments reported in the international medical literature. Increasing PPI dosage or the use of surgical therapy should be adopted in patients with insufficient control of acid excess.51,52 Several attempts to use add-on therapies to PPIs have obtained good results, particularly those combining mucosal protection and acid inhibition, as shown in a few controlled studies. In patients with poor response to 8-week PPIs, switching to vonoprazan 20 mg/ die allowed to obtain an enhanced symptom control and faster healing of mucosal lesions, probably due to the longer-lasting acid suppression of the P-CAB,53,54 but these studies were uncontrolled.
In presence of esophageal functional disorders, pain modulators may be the best therapeutic option, but their efficacy has not been strongly demonstrated.26
Also, the prolonged exposure of the oesophagus to bile acids may be responsible for PPI refractoriness, because duodenal-gastro-oesophagal reflux has been shown in 65% of patients who continued to complain of GERD-related symptoms despite PPI treatment.55 The diagnosis of ambulatory duodenal-gastro-oesophagal reflux is difficult to obtain and the most used method remains the measurement of bilirubin levels as a surrogate marker for bile reflux by means of Bilitec, which is generally adopted in the majority of investigational studies in this field.56 The modern MII-pH is able to measure weakly alkaline reflux events, but this diagnostic approach has not been validated either in research or in clinical studies. Farré et al17 showed that a mix of acid and biliary salts induced more intercellular dilated spaces (DIS) on oesophagal mucosa compared with acid alone. The same authors observed a progressive reduction in transepithelial resistance (TEER) associated with an increase in the concentration of biliary salts; the same results were not recorded with acid alone.
IW-3718 is a novel, gastric-retentive, extended-release formulation of the bile acid sequestrant colesevelam, which is able to bind bile acids in the stomach and, thus, may reduce their backflow into the oesophagus. Vaezi et al57 performed a randomized clinical trial to evaluate the efficacy and safety of different doses of IW-3718 as an adjunct therapy to PPIs in patients who showed lack of, or only partial, response to PPI treatment and found that this combination, given for 8 weeks, significantly reduced heartburn symptoms compared with adding placebo to PPIs. The dose of 1500 mg/daily of IW-3718 resulted to achieve the best results and was well tolerated. The mean change from baseline to week 8 in weakly heartburn severity score was the reduction of 46% in the placebo group and 58% in the 1500 mg IW-3718 and the mean change in weakly regurgitation frequency score in the same time period in the group with the active drug was a reduction of 17.5% compared with placebo. These findings suggest that IW-3718 may provide a further therapeutic option in improving both reflux typical symptoms in patients with refractory GERD, an important area of unmet need.
Neutralization of the Acid Pocket
Acid reflux episodes usually occur in the post-prandial periods,58 although intragastric pH is high because of the buffering effect of meals.59 This paradoxical phenomenon was first observed by Fletcher et al,60 who found that the average pH in the body of the stomach was remarkably higher (4.7 units) than the pH of the oesophagal refluxate (1.6 units). They explained this great difference in pH values by identifying a pocket of unbuffered gastric acid immediately below the EGJ. This phenomenon is exclusively post-prandial and can be found in both normal subjects and GERD patients. Moreover, it becomes supra-diaphragmatic more in reflux patients than in healthy individuals, particularly when a large hiatal hernia is present.61 Several studies have shown that alginate, a hetero-polysaccharide extracted from an ocean seaweed, is able to neutralize or displace the acid contained in the pocket and is ready to backflow into the oesophagus.62 This action contributes to justifying the post-prandial intake of alginate used as anti-reflux therapy.
Potassium Competitive Acid Blockers (P-CABs)
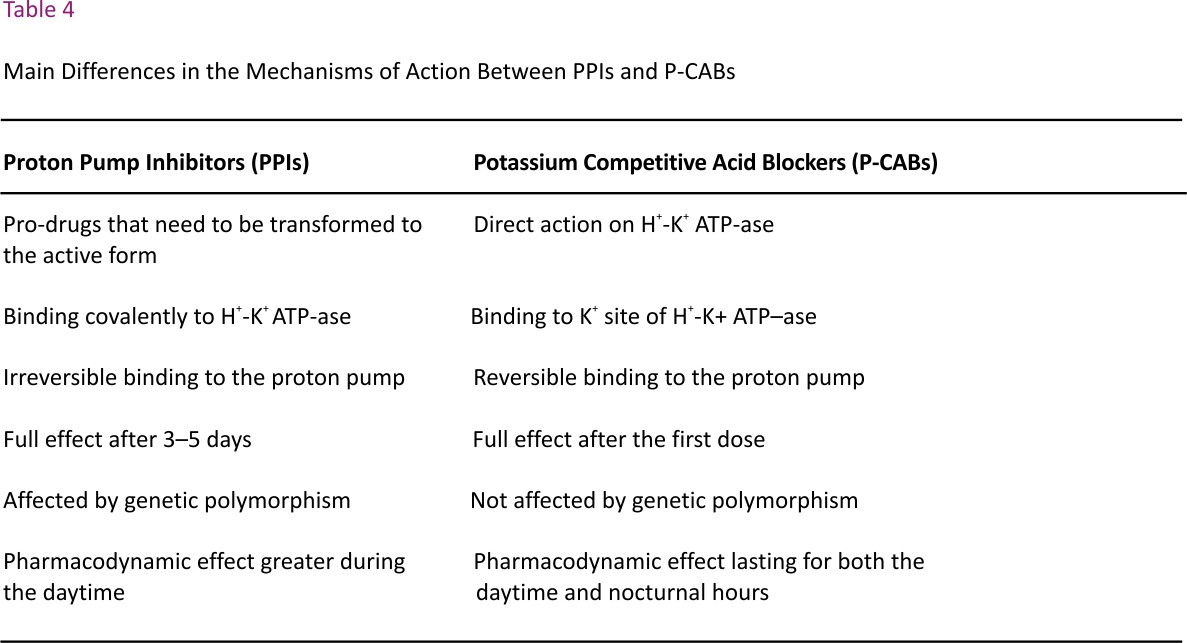
These novel antisecretory drugs differ from PPIs because they compete with K+ and induce a selective and reversible inhibition of the proton pump in a dose-dependent manner.63 They are not pro-drugs that must be activated in the parietal cells, like PPIs, and therefore their onset of action is immediate and the control of gastric acid secretion occurs after the first dose and within the first day of administration.64 Moreover, their dissociation rate from the proton pump is slow and its retention time in the gastric mucosa is 24 hours or more, thus the acid inhibitory activity covers both daytime and nighttime,65 differently from PPIs which are less effective during the nocturnal period.66 The main differences in the mechanisms of action between P-CABs and PPIs are reported in Table 4.
There are many molecules pertaining to this drug category (veraprazan, linaprazan, vonoprazan, tegoprazan, etc.), but vonoprazan is certainly the most studied in the treatment of GERD. It is marketed mainly in Asian countries, such as Japan, China, South Korea, Taiwan and Malaysia and Phase III studies are in progress in Europe and US. This drug has been shown to be effective and not inferior to PPIs in patients with mild or moderate degrees of EE,67 and its healing rate was even significantly better than that of lansoprazole in patients with the grades C and D of esophagitis,68 a superiority maintained in CYP2C19 metabolizers.69 This drug has also been shown to induce lower recurrence rates of esophagitis than lansoprazole when used as maintenance therapy.70 However, GERD symptom relief with vonoprazan 20 mg/daily did not differ from that obtained with esomeprazole 20 mg/ daily, even though this effect appeared more quickly.71 It is important to emphasize that significantly more patients with esophagitis achieved a complete resolution of nocturnal heartburn with vonoprazan than with lansoprazole, due to its prolonged ability to keep intragastric pH >4.0 units during the nighttime.72 Finally, the safety of vonoprazan does not differ from that of PPIs in a meta-analysis by Cheng et al.73 However, the remarkable acid suppression induced by vonoprazan needs careful control of possible adverse events, particularly in patients treated in the long term. In fact, the short-term safety of this drug is good and comparable with that of PPIs, while the chronic use of vonoprazan 10 mg and 20 mg daily over 52 weeks in patients treated to prevent reflux esophagitis recurrence determined a progressive increase of serum gastrin up to 678 pg/mL on average, with the higher dose, even though there were no relevant effects on gastric neuroendocrine cells.69 As to other adverse events, changes in the gut microbiome have been documented with vonoprazan, thus increasing the risk of enteric infections in patients travelling to tropical areas.
Reflux Inhibitors
It is well known that TLESRs represent the most relevant mechanism in the pathophysiology of GERD and therefore its control has become a therapeutic target in the therapy of this disease. Baclofen, a gamma-amino-butyric acid (GABA) receptor type B agonist, has been identified as the first reflux inhibitor and, as such, is able to reduce the number of TLESRs and all types of reflux events, both acid and weakly acidic, as shown by means of impedance-pH monitoring.74 A meta-analysis of nine studies has found that baclofen decreased the number and the length of reflux episodes as well as the incidence of TLESRs.75 However, its clinical use is very limited because of its poor tolerability due mainly to neurological adverse events.76 Other similar agents (lesogaberan and arbaclofen placarbil) did not show a relevant therapeutic efficacy compared with placebo and their development has been stopped.77
Enhancing Esophageal Clearance and Defensive Properties
We have already said that GERD patients exhibit a greater volume of refluxate and longer acid clearance times than normal subjects and therefore limiting the contact time between refluxate and oesophagal mucosa by improving peristaltic function may be a useful therapeutic attempt. Moreover, reducing the mucosal permeability by means of drugs able to reinforce the defensive properties of the oesophagal lining, thus blocking the toxic effect of the gastric substances contained in the refluxate, is a further potentially adequate therapeutic proposal.
Prokinetics
A list of currently used prokinetic compounds is reported in Table 5. These drugs have the potential to enhance oesophagal clearance of refluxate by stimulating valid peristalsis and accelerating gastric emptying. Although these possible good actions aimed at reducing the contact time of refluxate with oesophagal mucosa and preventing the backflow of meals and secretions retained for more time than usual in the stomach, there is no high-quality evidence for their use in GERD patients, as either monotherapy or adjunctive therapy.78 Not surprisingly, the US guidelines published in 2018 do not recommend these drugs as a therapeutic option for PPI-refractory GERD patients.79 In addition, almost all prokinetic agents available present a certain risk of cardiac toxicity (mainly arrhythmias) or neurologic adverse events.80 In fact, many of these agents approved for the management of GI motility disorders carry a small but increased risk of drug-induced arrhythmia. Epidemiologic studies have identified many important patient-specific and drug-specific risk factors that, when present, typically in combination, exponentially increase the risk of drug-induced long QT syndrome, which is associated with the great risk of cardiac arrest and death.
Only prucalopride was proven to be safe from the cardiac standpoint, thanks to its high selectivity for 5-HT4 receptors,81 and enables to accelerate gastric emptying and reduce AET, but clinical trials assessing its efficacy in GERD treatment are lacking.
Mucosal Protection
Many studies have recently shown that impaired mucosal integrity is involved in the pathogenesis of GERD and in the generation of typical symptoms, particularly heartburn. In the last years, mucosal baseline impedance measured by the modern impedance-pH monitoring has emerged as a novel method to assess the alterations of mucosal integrity in GERD patients.82,83 A recent study has shown that this metric varies with the GERD phenotype because it tends to decrease from FH to NERD and, even more, in EE, thus confirming the value of mucosal integrity as a marker of weakened mucosal protection and opening a new avenue for GERD treatment.84 In addition, an elegant study by Woodland et al85 has demonstrated that the intramucosal distribution of nerve fibres is more superficial in NERD than in EE and Barrett’s oesophagus and this provides a reasonable explanation for the higher sensitivity of the first population toward all substances bathing the mucosa of the organ.
Among the few compounds displaying a mucosal protective activity, there is alginate, which was found to coat in vitro the luminal surface of oesophagal mucosa for about 1 hour86 and in vivo, this protective effect was shown to be as long as 10 min, on average.87 This long-lasting adhesion is particularly relevant in relation to the fast transit time of liquids through the oesophagus (less than 16 s), even in a supine position.88
The association of mucosal protection with acid inhibition with PPI has been assessed in a randomized clinical trial in NERD patients and resulted to achieve a percentage of heartburn-free days significantly higher than that of PPI alone.89 Moreover, the use of alginate as add-on therapy to PPIs in GERD patients with partial response to the latter drugs was shown to relieve heartburn and to ameliorate the quality of life significantly better than PPIs alone.90 Therefore, the bio-adhesive properties of alginate permit to improve the success of PPIs in the treatment of GERD, when these two compounds are used combined in both NERD patients and in those who are partially unresponsive to these powerful antisecretory drugs.
A new medical device containing hyaluronic acid and chondroitin-sulfate (EsoxxTM, Alfasigma, Italy) has been developed as an oesophagal protective agent. The European Council classified this formulation as a class III medical device, which should be used in human beings for the purpose of treatment or alleviation of disease. It is dispersed in a bio-adhesive carrier (poloxamer 407), which prolongs its residence time in the lumen and creates a mechanical barrier against noxious agents of refluxate over the oesophagal lining.91 Both compounds exhibit multiple functions, such as anti-inflammatory effect, wound repair, tissue regeneration and modulation of cytokines expression.16 A prospective, randomized clinical trial performed in Italy in NERD patients compared EsoxxTM combined with a standard PPI dose to PPI plus placebo.92 The combined therapy protracted for 14 days was significantly better than the latter one in relieving symptoms and improving the quality of life of recruited patients. The treatment was well-tolerated and no serious adverse events were registered.
Visceral Hypersensitivity
The Committee of Rome IV criteria for esophageal functional disorders sustained that both RH and FH have to be included in this category and only NERD patients with abnormal AET pertain to the population with GERD.24 This new classification was based on the assumption that RH and FH are due to visceral hypersensitivity, without taking under consideration that the former is characterized by a positive association between symptoms and reflux episodes, although the oesophagal acid exposure is normal, whereas the latter does not exhibit any relationship of this type.
Even though the reduction of visceral hypersensitivity is a reasonable therapeutic target in these functional patients, the results of clinical trials using neuromodulators (Table 6) have provided conflicting findings, because some studies have shown the benefit of these drugs,93,94 while others did not find any difference between anti-depressants, particularly tricyclic compounds at low dosage, and placebo.95,96 On the contrary, surgical therapy has provided promising results in both uncontrolled97–99 and controlled clinical studies100 performed in patients with RH. These good results were also maintained over a follow–up of 3–5 years.98,101 These findings contribute to question the above-mentioned Rome IV criteria and support the need of maintaining RH and FH as separate entities, thus re-classifying most RH patients within the GERD spectrum25,26 in order to adopt the right therapeutic decisions.
Conclusions
PPIs remain the first-choice therapy in both short- and long-term medical treatment for EE due to their well-documented benefit in controlling heartburn, healing esophagitis and preventing disease recurrences. Despite their effectiveness, there is a consistent proportion of GERD patients who respond only partially to them and the majority pertain to the NERD population, particularly the RH and FH subgroups. It is mandatory to test these patients in order to understand the various reasons for PPI failure and accordingly to adopt the most useful treatments instead of managing them empirically with frequent unsuccessful and potentially dangerous attempts.
PPIs present several drawbacks, such as the slow onset of action, short bio-availability, insufficient control of nocturnal acid secretion, which can impair their benefit. In the last decade, a new class of acid-suppressive agents, the P-CABs, have been introduced on the market, especially in Asian countries, and have been shown to be successful in the treatment of GERD, because they seem to be able to overcome some PPI pitfalls, but more clinical comparative studies are needed in North America and European populations with the various manifestations of GERD. In patients with NERD, the benefit of PPIs is lower because of their pathophysiological heterogeneity, because oesophagal hypersensitivity seems to prevail on the damage due to acid. However, the results of the few randomized clinical trials using the current pain modulators are far from being satisfactory.
Expert Opinion
PPIs remain the standard therapeutic approach to GERD patients, although 20%-40% of them do not respond adequately to these antisecretory drugs. The majority of PPI non-responders pertain to the NERD population, but also up to 15% of patients with EE do not achieve full remission of their inflammatory lesions after at least 8 weeks of treatment. This high proportion of GERD patients with incomplete or null responses to PPIs represents the most challenging population for physicians. Nowadays it is recommended to investigate them with appropriate examinations in order to understand why they continue to have persistent symptoms instead of managing them empirically with the risk of increased costs and adverse events using long-lasting and higher than usual doses of PPIs.
Endoscopy is useful to rule out other causes of esophagitis (pill-induced esophagitis, eosinophilic esophagitis, etc.), but ambulatory impedance-pH monitoring and high-resolution manometry have become the tests of choice to identify patients with excess acid despite PPI therapy or those with symptoms not due to GERD or finally, those with motility disorders who can manifest the same disturbances of GERD patients.
In cases with partial response to PPIs, several clinical studies have shown that a consistent therapeutic gain can be achieved by combining PPIs with mucosal protective drugs (alginate, formulations containing hyaluronic acid and chondroitin-sulfate) or recently with a bile acid sequestrant drug. In alternative to PPIs, also the use of vonoprazan, the most studied among the antisecretory drugs blocking the K+ exchange channel of the proton pump (P-CABs), has been found to increase the rate of response in patients with persistent symptoms despite taking PPIs. It must be stressed that prokinetics, which continue to be used frequently by many physicians, are devoid of any therapeutic help in the treatment of GERD, both alone and in co-prescription with PPIs.
P-CABs are interesting drugs for future use because they have shown a faster onset of action and a longer-lasting increase of intragastric pH compared with PPIs so that they can be used in alternative to the later ones in order to improve the healing rates of EE, particularly the most severe grades (C and D) of erosive lesions. They have been used so far mainly in Asian countries and should be more evaluated in western populations, especially in patients with NERD.
Finally, greater attention than in the past has been paid in last years toward the use of drugs able to protect oesophagal mucosa and reinforce its defensive properties, a therapeutic target that has been overlooked for a long time. These drugs have been found to be not inferior to PPIs in relieving GERD symptoms and improving the quality of life of NERD patients, as either monotherapy or combined with PPIs. They seem to have opened a new avenue in the continuous search for medications able to solve the actual unmet needs of medical anti-reflux therapy.
However, the future of GERD management, particularly for challenging cases, will not rely on the development of new drugs, but on the better identification by means of the objective diagnostic tests available (impedance-pH monitoring, Bravo system and high-resolution manometry) of patients with suspected symptoms of GERD or those who do not respond satisfactorily to standard antisecretory therapy with PPIs. Last but not least is the appropriate indication for surgical therapy, which remains the only approachable to control the pathophysiological alterations leading to the development of GERD.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vakil N, van Zanten SV, Kahrilas P, al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101: 1900–1920. doi:10.1111/j.1572-0241.2006. 00630.x
2. Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastroesophageal reflux disease: a systematic review. Gut. 2005;54: 710–717. doi:10.1136 /gut.2004.051821
3. Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi:10. 1053/j.gastro.2005.12. 016
4. Savarino E, Zentilin P, Marabotto E, et al. Overweight is a risk factor for both erosive and non-erosive reflux disease. Dig Liver Dis. 2011; 43: 940– 945. doi:10.1016/j.dld.2011.07.014
5. Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenteroet l Motil.2015;27: 1202– 1213. doi:10.1111/nmo. 12611
6. Savarino E, de Bortoli N, De Cassan C, et al. The natural history of gastro-oesophagal reflux disease: a comprehensive review. Dis Esophagus. 2017;30:1–9. doi:10.1111/dote. 12511 *Exhaustive review on the evolution of GERD overtime supporting the good prognosis of the disease
7. Savarino V, Marabotto E, Zentilin P, et al. Pathophysiology, diagnosis, and pharmacological treatment of gastro-oesophagal reflux disease. Expert Rev Clin Pharmacol. 2020; 13:437–449. doi:10.1080/1751 2433.2020.1752664
8. Pouderoux P, Verdier E, Kahrilas PJ. Patterns of oesophagal inhibition during swallowing, pharyngeal stimulation, and transient LES relaxation. Lower oesophagal sphincter. Am J Physiol Gastrointest Liver Physiol. 2003;284: G242 –G24 7. doi:10.1152/ajpgi.00301.2002
9. Savarino E, Mei F, Parodi A, et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology. 2013;52:1095– 1100. doi:10.1093/rheumatology /kes429
10. Tolone S, Savarino E, Zaninotto G, et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: a comparison with surgical in vivo evaluation. United European Gastroenterol J. 2018; 6:981–989. doi:10.1177/2050640 618769160
11. Savarino E, Bredenoord AJ, Fox M, et al. International working group for disorders of gastrointestinal motility and function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14: 665–676. doi:10.1038/nrgastro. 2017.130 **Important international consensus on the advancements of diagnostic tools for the diagnosis of GERD
12. Frazzoni M, de Bortoli N, Frazzoni L, et al. Impedance-pH monitoring for diagnosis of reflux disease: new perspectives. Dig Dis Sci. 2017;62: 1881–1889. doi:10.1007/s10620-017-4625-8
13. Nobile S, Meneghin F, Marchionni P, et al. Response to therapy among neonates with gastro-oesophagal reflux is associated with oesophagal clearance. Early Hum Dev. 2021; 152:105248. doi:10.1016/j.earl humdev.2020. 105248
14. McCallum RW, Berkowitz DM, Lerner E. Gastric emptying in patients with gastroesophageal reflux. Gastroenterology. 1981;80:285– 291. doi:10.1016/0016-5085(81) 90716-2
15. Zentilin P, Dulbecco P, Bilardi C, et al. Circadian pattern of intragastric acidity in patients with non-erosive reflux disease (NERD). Aliment Pharmacol Ther. 2003;17:353–359. doi:10. 1046/j.1365-2036.2003. 01422.x
16. Scarpignato C, Hongo M, Wu JCY, et al. Pharmacologic treatment of GERD: where we are now, and where are we going? Ann N Y Acad Sci. 2020.
17. Farré R. Pathophysiology of gastro-oesophagal reflux disease: a role for mucosa integrity? Neurogastro- enterol Motil. 2013;25:783–799. doi:10.1111/nmo.12201
18. Zentilin P, Savarino V, Mastracci L, et al. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100: 2299–2306. doi:10.1111/j.1572-0241.2005.50209.x
19. Savarino E, Zentilin P, Mastracci L, et al. Microscopic esophagitis distinguishes patients with non-erosive reflux disease from those with functional heartburn. J Gastroenterol. 2013; 48:473–482. doi:10.1007/s00535-012-0672-2 **Prospective study on the different distribution of microscopic esophagitis among the various subgroups of GERD
20. Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013; 10:371– 380. doi:10.1038/ nrgastro. 2013.50
21. Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40: 275–285. doi:10.1080 /00365520510011579
22. Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354– 1359. doi:10.1136/gut.2007 .145177
23. Savarino E, Zentilin P, Marabotto E, et al. A review of pharmacotherapy for treating gastroesophageal reflux disease (GERD). Expert Opin Pharmacother. 2017;18:1333–1343. doi:10.1080/14656566. 2017. 1361407
24. Aziz Q, Fass R, Gyawali CP, et al. Functional oesophagal disorders. Gastroenterology. 2016; 150(6):1368–1379. doi:10.1053/j. gastro. 2016.02.012 **The most recent consensus on the classification of esophageal functional disorders
25. Frazzoni L, Frazzoni M, de Bortoli N, et al. Critical appraisal of Rome IV criteria: hypersensitive oesophagus does belong to gastroesophageal reflux disease spectrum. Ann Gastroenterol. 2018;31:1–7. doi:10.20524/aog.2017.0199
26. Savarino V, Marabotto E, Zentilin P, et al. Esophageal reflux hypersensitivity: non-GERD or still GERD? Dig Liver Dis. 2020;52:1413– 1420. doi:10. 1016/j.dld.2020.10.003
**Updated review disputing the inclusion of reflux hypersensitivity in the group of esophageal functional disorders
27. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11: 1123–1134. doi:10.1080/17512433.2018. 1531703
28. Savarino V, Di Mario F, Scarpignato C. Proton pump inhibitors in GORD. An overview of their pharmacology, efficacy and safety. Pharmacol Res.2009;59:135–153. doi:10.1016/j.phrs. 2008.09.016
29. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45: 172–180. doi:10.1136/gut. 45.2.172
30. Khan M, Santana J, Donnellan C, et al. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007; CD003244.
31. van Pinxteren B, Sigterman KE, Bonis P, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2010:CD002095. doi:10.1002/14651858. CD002095.pub4
32. Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011; 106:1419–1425. doi:10.1038/ajg. 2011.146
33. Kahrilas PJ, Hughes N, Howden CW. Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastro-oesophageal reflux disease. Gut. 2011;60:1473–1478. doi:10.1136/gut.2011. 241307
34. Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2004;2: 665–668. doi:10.1016 /S1542-3565(04)00289-7
35. Dean BB, Gano AD, Knight K, et al. Effectiveness of proton pump inhibitors in non-erosive reflux disease. Clin Gastroenterol Hepatol. 2004;2: 656–664. doi:10.1016/S1542-3565(04) 00288 -5
36. Scarpignato C. Poor effectiveness of proton pump inhibitors in non-erosive reflux disease: the truth in the end! Neurogastroenterol Motil. 2012;24:697–704. doi:10.1111/j.1365-2982. 2012.01977.x
37. Weijenborg PW, Cremonini F, Smout AJ, et al. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012; 24:747–757. doi:10.1111/j.1365-2982.2012.01 888.x
38. Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med.1995;333: 1106–1110. doi:10.1056/NEJM199510263 331703
39. Thjodleifsson B, Rindi G, Fiocca R, et al. European Rabeprazole Study Group. A randomized, double-blind trial of the efficacy and safety of 10 or 20 mg rabeprazole compared with 20 mg omeprazole in the maintenance of gastro-oesophageal reflux disease over 5 years. Aliment Pharmacol Ther. 2003;17:343–351. doi:10. 1046/j.1365-2036. 2003.01 446. x
40. Ghisa M, Della Coletta M, Barbuscio I, et al. Updates in the field of non-oesophagal gastroesophageal reflux disorder. Expert Rev Gastroenterol Hepatol. 2019;13:827–838. doi:10.1080/17474124.2019.1645593
41. Savarino V, Dulbecco P, Savarino E. Are proton pump inhibitors really so dangerous? Dig Liver Dis. 2016;48: 851–859.
42. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152: 706–715. doi:10.1053/j. gastro.2017.01.031 **Important review questioning the recent publications on the many presumed adverse events linked to chronic PPI therapy
43. Savarino V, Marabotto E, Furnari M, et al. Latest insights into the hot question of proton pump inhibitor safety – a narrative review. Dig Liver Dis. 2020;52:842–852. doi:10.1016/j.dld.2020. 04.020
44. El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32: 720–737. doi:10. 1111/j.1365-2036.2010. 04406.x
45. Katz PO. The proton pump inhibitor is not working: assess don’t guess. Gastroenterology. 2021; 160 (1): 19–20. doi:10.1053/j.gastro. 2020.10.043
46. Savarino E, Pohl D, Zentilin P, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009;58:1185–1191. doi:10.1136/gut. 2008.175810
47. Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61: 1340– 1354. doi:10.1136/gutjnl-2011-301897
48. Penagini R, Sweis R, Mauro A, et al. Inconsistency in the diagnosis of functional heartburn: usefulness of prolonged wireless pH monitoring in patients with proton pump inhibitor refractory gastroesophageal reflux disease. J Neurogastroenterol Motil. 2015;21:265–272. doi:10. 5056/jnm14075
49. Frazzoni M, de Bortoli N, Frazzoni L, et al. The added diagnostic value of post reflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil. 2017; 29(3):e12947. doi:10.1111/nmo. 12947
50. de Bortoli N, Frazzoni L, Savarino EV, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. Am J Gastroenterol. 2016;111: 1711–1717. doi:10. 1038/ajg.2016.432
51. Watson RG, Tham TC, Johnston BT, et al. Double-blind cross-over placebo-controlled study of omeprazole in the treatment of patients with reflux symptoms and physiological levels of acid reflux–the “sensitive oesophagus”. Gut. 1997;40:587 –590. doi:10.1136/gut.40.5.587
52. Pauwels A, Boeckxstaens V, Andrews CN, et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut. 2019;68: 1928–1941. . doi:10.1136/gutjnl-2019-318260 **Important international consensus on the criteria to select the right adult patients as candidates for anti-reflux surgery
53. Hoshino S, Kawami N, Takenouchi N, et al. Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion. 2017; 95:156–161. doi:10.1159/00045 6072
54. Akiyama J, Hosaka H, Kuribayashi S, et al. Efficacy of vonoprazan, a novel potassium-competitive acid blocker, in patients with proton pump inhibitor-refractory acid reflux. Digestion. 2020;101(2):174–183. doi:10.1159/00049 7775
55. Tack J, Koek G, Demedts I, et al. Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett’s oesophagus: acid reflux, bile reflux, or both? Am J Gastroenterol. 2004;99:981–988. doi:10.1111/j.1572-0241.2004. 04171.x
56. de Bortoli N, Gyawali CP, Frazzoni M, et al. Bile reflux in patients with NERD is associated with more severe heartburn and lower values of mean nocturnal baseline impedance and chemical clearance. Neurogastro- enterol Motil. 2020;32:e13919. doi:10.1111/nmo.139 1 9
57. Vaezi MF, Fass R, Vakil N, et al. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a randomized trial. Gastroenterology. 2020; 158:2093 –2103. doi:10.1053/j.gastro.2020. 02.031
58. Burgerhart JS, van de Meeberg PC, Siersema PD, et al. Nocturnal and daytime oesophagal acid exposure in normal-weight, overweight, and obese patients with reflux symptoms. Eur J Gastroenterol Hepatol. 2014;26:6–10. doi:10. 1097/MEG. 0b013e328365c3cb
59. Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol. 2013;108:1058–1064. doi:10. 1038/ajg.2013.132
60. Fletcher J, Wirz A, Young J, et al. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology. 2001;121:775–783. doi:10.1053/gast.2001. 27997
**First study reporting the pathophysiological value of the acid pocket as a factor favouring postprandial reflux
61. Beaumont H, Bennink RJ, de Jong J, et al. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut. 2010;59:441–451. doi:10. 1136/gut.2009.178061
62. Kwiatek MA, Roman S, Fareeduddin A, et al. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment Pharmacol Ther. 2011; 34:59–66. doi:10.1111/j.1365-2036.2011. 04678.x
63. Martinucci I, Blandizzi C, Bodini G, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother. 2017; 18:1145–1152. doi:10. 1080/1465 6566. 2017.1346087
64. Savarino E, Martinucci I, Furnari M, et al. Vonoprazan for treatment of gastroesophageal reflux: pharmacodynamic and pharmacokinetic considerations. Expert Opin Drug Metab Toxicol. 2016;12:1333–1341. doi:10. 1080/174 25255.2016.1214714
65. Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/ non-Japanese subjects. Clin Transl Gastroenterol. 2015; 6:e94. doi:10.1038/ctg. 2015.18
66. Savarino V, Mela GS, Zentilin P, et al. Comparison of 24-h control of gastric acidity by three different dosages of pantoprazole in patients with duodenal ulcer. Aliment Pharmacol Ther. 1998; 12:1241–1247. doi:10.1046/j.1365-2036. 1998.00416.x
67. Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Therap Adv Gastroenterol. 2018; 11:1756283X 17745776. doi:10. 1177/1756283X17745776
68. Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015; 42:685–695. doi:10.1111/apt.13331
69. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43:240–251. doi:10. 1111/apt.13461
70. Ashida K, Iwakiri K, Hiramatsu N, et al. Maintenance for healed erosive esophagitis: phase III comparison of vonoprazan with lansoprazole. World J Gastroenterol. 2018; 24:1550– 1561. doi:10.3748/wjg.v24.I14. 1550
71. Sakurai K, Suda H, Fujie S, et al. Short-term symptomatic relief in gastroesophageal reflux disease: a comparative study of esomeprazole and vonoprazan. Dig Dis Sci. 2019; 64:815–822. doi:10.1007/s10620-018-5365-0
72. Oshima T, Arai E, Taki M, et al. Randomised clinical trial: vonoprazan versus lansoprazole for the initial relief of heartburn in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:140–146. doi:10.1111/apt. 15062
73. Cheng Y, Liu J, Tan X, et al. Direct comparison of the efficacy and safety of vonoprazan versus proton-pump inhibitors for gastroesophageal reflux disease: a systematic review and meta-analysis. Dig Dis Sci. 2020. doi:10.1007/s10620-020-06141-5
*Updated systematic review and meta-analysis comparing the novel most studied P-CAB vonoprazan and traditional PPIs
74. Vela MF, Tutuian R, Katz PO, et al. Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther. 2003;17: 243–251. doi:10.1046/j.1365-2036.2003. 01394.x
75. Li S, Shi S, Chen F, et al. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;307805.
76. Wise J, Conklin JL. Gastroesophageal reflux disease and baclofen: is there a light at the end of the tunnel? Curr Gastroenterol Rep. 2004; 6:213– 219. doi:10.1007/s11894-004-0010-9
77. Boeckxstaens GE, Denison H, Jensen JM, et al. Translational gastrointestinal pharmacology in the 21st century: ‘the lesogaberan story’. Curr Opin Pharmacol. 2011;11:630–633. doi:10. 1016/j.coph.2011.10.011
78. Ren LH, Chen WX, Qian LJ, et al. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol.2014;20:2412–2419. doi:10. 3748/wjg.v20.i9.2412
79. Yadlapati R, Vaezi MF, Vela MF, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol. 2018; 113:980–986. doi:10. 1038/s41395-018-0045-4
80. Giudicessi JR, Ackerman MJ, Camilleri M. Cardiovascular safety of prokinetic agents: a focus on drug-induced arrhythmias. Neurogastroenterol Motil. 2018;30:e13302. doi:10. 1111/nmo.13302
81. Tack J, Corsetti M. Prucalopride: evaluation of the pharmacokinetics, pharmacodynamics, efficacy and safety in the treatment of chronic constipation. Expert Opin Drug Metab Toxicol. 2012;8:1327–1335. doi:10.1517/17425255. 2012.719497
82. Roman S, Gyawali CP, Savarino E, et al. GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-oesophagal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil.2017;29: 1–15. doi:10.1111/nmo. 13067
**Updated international consensus on the use of ambulatory impedance-pH monitoring for the diagnosis of GERD
83. Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. 2016;14:40–46. doi:10.1016/j.cgh. 2015.06.026
84. Frazzoni M, de Bortoli N, Frazzoni L, et al. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive oesophagus from functional heartburn. J Gastroenterol. 2017;52:444–451. doi:10.1007/s00535-016-1226-9
**Important paper highlighting the role of the new metrics derived from impedance-pH tracings in distinguishing reflux hypersensitivity from functional heartburn
85. Woodland P, Shen Ooi JL, Grassi F, et al. Superficial oesophagal mucosal afferent nerves may contribute to reflux hypersensitivity in non-erosive reflux disease. Gastroenterology. 2017;153: 1230–1239. doi:10.1053/j.gastro. 2017.07.017
86. Woodland P, Batista-Lima F, Lee C, et al. Topical protection of human oesophagal mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2015;308(12):G975–G980. doi:10.1152/ajpgi. 00424.2014
87. Sonmez S, Coyle C, Sifrim D, et al. Duration of adhesion of swallowed alginates to distal oesophageal mucosa: implications for topical therapy of oesophageal diseases. Aliment Pharmacol Ther. 2020;52: 442–448. doi:10. 1111/apt.15884
**Relevant study calculating in vivo the average time of adhesion of alginate to the oesophagal mucosa
88. Blackshaw LA, Bordin DS, Brock C, et al. Pharmacologic treatments for oesophagal disorders. Ann N Y Acad Sci. 2014;1325:23–39. doi:10.1111/ nyas.12520
89. Manabe N, Haruma K, Ito M, et al. Efficacy of adding sodium alginate to omeprazole in patients with non-erosive reflux disease: a randomized clinical trial. Dis Esophagus. 2012;25: 373– 380. doi:10.1111/j.1442-2050. 2011.01276.x
90. Reimer C, Lødrup AB, Smith G, et al. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once-daily proton pump inhibitor. Aliment Pharmacol Ther. 2016;43:899–909. doi:10.1111/apt. 13567
91. Di Simone MP, Baldi F, Vasina V, et al. Barrier effect of Esoxx(®) on oesophagal mucosal damage: an experimental study on ex-vivo swine model. Clin Exp Gastroenterol.2012;5:103–107. doi:10. 2147/CEG.S31 404
92. Savarino V, Pace F, Scarpignato C; Esoxx Study Group. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease – efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017;45:631–642. . doi:10.1111/apt.13914 *Prospective study evaluating the benefit of PPIs combined with a new medical device protecting oesophagal mucosa in the resolution of GERD symptoms
93. Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive oesophagus: a randomized, double-blind, placebo-controlled study. Am J Gastro enterol.2012;107:1662–1667. doi:10.1038/ajg.2011.179
94. Ostovaneh MR, Saeidi B, Hajifathalian K, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once-daily proton pump inhibitors: a double-blind placebo-controlled trial. Neurogastroenterol Motil.2014;26:670 –678. doi:10. 1111/nmo.12313
95. Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, et al. Imipramine for treatment of oesophagal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol.2016;111:217–224. doi:10.1038/ ajg.2015.413
96. CM, Tomiozzo JC, Farré R, et al. Effect of nortriptyline on brain responses to painful oesophagal acid infusion in patients with non-erosive reflux disease. Neurogastroenterol Motil. 2014;26:187–195. doi:10.1111 /nmo.12251
97. Mainie I, Tutuian R, Agrawal A, et al. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg. 2006;93: 1483–1487. doi:10.1002/ bjs.5493
98. Broeders JA, Draaisma WA, Bredenoord AJ, et al. Oesophageal acid hypersensitivity is not a contraindication to Nissen fundoplication. Br J Surg. 2009;96:1023–1030. doi:10.1002/bjs.66 84
99. Frazzoni M, Conigliaro R, Melotti G. Reflux parameters as modified by laparoscopic fundoplication in 40 patients with heartburn /regurgitation persisting despite PPI therapy: a study using impedance-pH monitoring. Dig Dis Sci. 2011;56:1099–1106. doi:10.1007/s10620-010-1381-4
100. Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381: 1513–1523. doi:10.1056/ NEJMoa 1811424
**Important prospective study showing the benefit of surgical therapy in patients with reflux hypersensitivity
101. Frazzoni M, Piccoli M, Conigliaro R, et al. Refractory gastroesophageal reflux disease as diagnosed by impedance-pH monitoring can be cured by laparoscopic fundoplication. Surg Endosc. 2013;27:2940–2946. doi:10.1007/ s00464-013-2861-3
Credits: Savarino, V., Marabotto, E., Zentilin, P., Demarzo, M. G., de Bortoli, N., & Savarino, E. (2021). Pharmacological Management of Gastro-Esophageal Reflux Disease: An Update of the State-of-the-Art. Drug design, development and therapy, 15, 1609–1621. https://doi.org/10.2147/DDDT.S306371