Sebastian Schwab,1 Daniel Pörner,1 Carola-Ellen Kleine,1 Roxana Werberich,1 Louisa Werberich,1 Stephan Reinhard,1 Dominik Bös,2 Christian P. Strassburg,1 Sibylle von Vietinghoff,1 Philipp Lutz,#1 and Rainer P. Woitas#3
Contact Sebastian Schwab – 1Department of Internal Medicine I, Nephrology Section, University of Bonn, Bonn, Germany
Abstract
Background
For the improvement of outcomes after renal transplantation, it is important to predict the future risk of major adverse cardiac events as well as all-cause mortality. We aimed to determine the relationship of pre-transplant NT-proBNP with major adverse cardiac events and all-cause mortality after transplant in patients on the waiting list with preserved left ventricular ejection fraction.
Patients and methods
We included 176 patients with end-stage renal disease and preserved left ventricular ejection fraction who received a kidney transplant. MACE was defined as myocardial infarction (ST-segment elevation [STEMI] or non-ST-segment elevation [NSTEMI]), stroke or transient ischemic attack), coronary artery disease requiring intervention or bypass, or death from cardiovascular causes.
Results
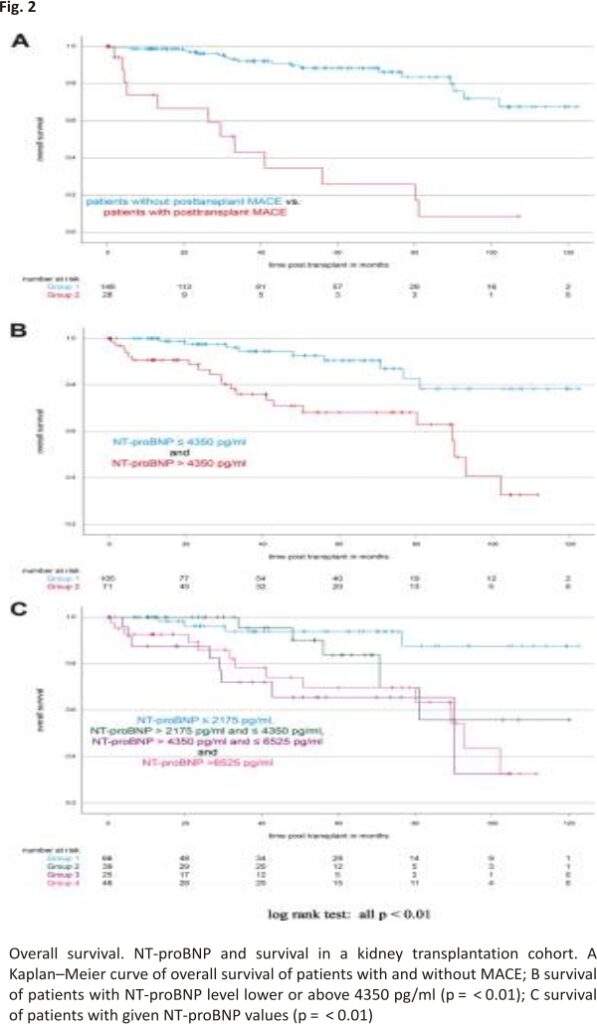
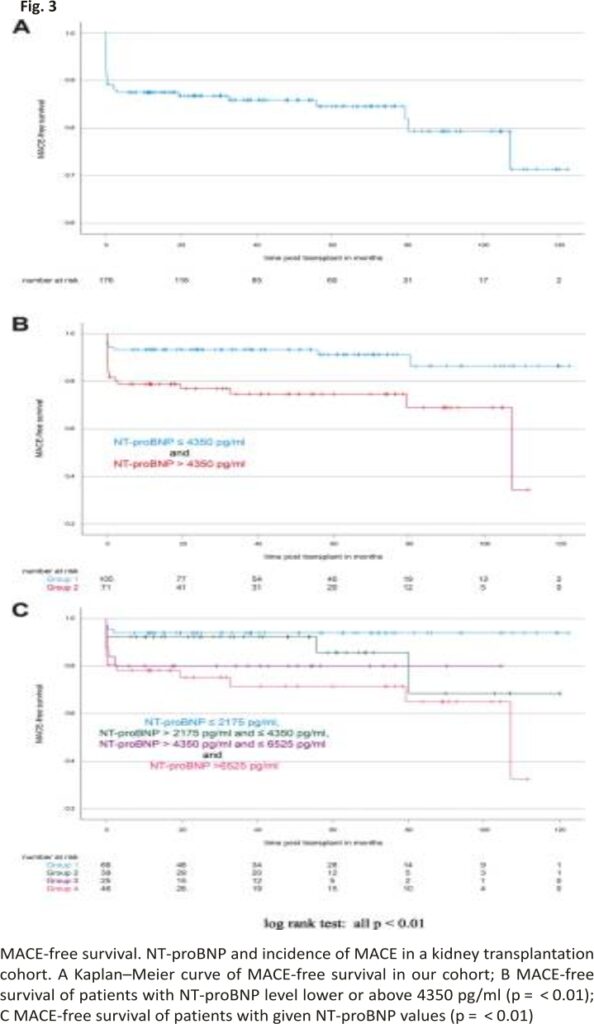
MACE occurred in 28/176 patients. Patients with NT-proBNP levels above 4350 pg/ml had 1- and 5-year survival rates of 90.67% and 68.20%, whereas patients with NT-proBNP levels below 4350 pg/ml had 1- and 5-year survival rates of 100% and 90.48% (p < 0.01). 1- and 5-year MACE-free survival rates were calculated as 78.82% and 74.68% for patients with NT-proBNP > 4350 pg/ml and 93.33% and 91.21% for patients with NT-proBNP < 4350 pg/ml (p < 0.01).
Conclusions
Pre-transplant NT-proBNP might identify renal transplant candidates at risk for MACE after transplant.
Keywords: NT-proBNP, Renal transplantation, Major cardiovascular event
Introduction
Renal transplantation is a treatment of end-stage kidney disease (ESKD), which largely improves the quality of life and prolongs survival 1. Recipients of a kidney transplant have a decreased incidence of cardiovascular events compared to patients remaining on the waiting list, whereas their cardiovascular risk is still higher than in the general population 2. To improve outcomes for renal transplant patients, estimation of future cardiovascular risk is therefore important. However, compared to the general population, risk assessment is inadequately delineated for patients with ESKD 3, especially for the period after kidney transplant in patients with preserved cardiac function. A widely available, simple biomarker to predict major adverse cardiac events (MACE) in renal transplant recipients would be very helpful to counsel patients and tailoring individual preventive strategies.
Amino-terminal pro-B-type natriuretic peptide (NT-proBNP) is a marker of myocardial damage and fluid volume overload 4,5. Asymptomatic and euvolemic patients on maintenance dialysis often show NT-proBNP plasma levels greater than 20 times of the upper limit 3,6. NT-proBNP predicts cardiovascular morbidity and all-cause death both in the general population and in chronic kidney disease 7–10. However, the value of NT-proBNP as a reliable cardiac marker in patients with renal failure has been questioned 11–13, because NT-proBNP is at least partially cleared from the circulation by the kidneys 14, 15.
NT-proBNP was identified as a risk factor for mortality with an even stronger association in a cohort of renal transplant recipients than in the general population 13. In spite of its well-known association with major cardiac events (MACE) and mortality in ESKD patients, it is largely unknown if this association holds true for patients once they received kidney transplantation. Only a few studies investigated this association. In contrast to the previous study 13, NT-proBNP was measured before transplantation in our cohort. Additionally, NT-proBNP at transplantation was found to be significantly higher in patients suffering from MACE very early after transplant, but this analysis comprised only a few patients and analyzed MACE only within the first days after transplant 16. Because cardiac insufficiency may be considered a risk factor for MACE and mortality, we focused on patients without impaired left ventricular ejection fraction.
To further clarify the relationship between NT-proBNP and risk for MACE, we investigated pre-transplantation NT-proBNP as a potential predictor of MACE and mortality in patients with preserved left ventricular ejection fraction following renal transplantation.
Patients and methods
Study population
This retrospective study was performed at the University Hospital Bonn, Germany. Patients suffering from ESKD and wait-listed for kidney transplantation were included if they were at least 17 years old at the time of kidney transplantation. NT-proBNP was measured during routine laboratory evaluation directly before kidney transplantation in every patient without a specific clinical event to trigger the analysis. Because the timepoint of analysis was determined by the availability of a donor organ, there was no specific interval to the dialysis sessions apart from the fact that specimens were not collected during dialysis. The period between NT-proBNP measurement and transplantation was no longer than 24 h.
For this study, only patients with pre-transplant measurement of NT-proBNP and with preserved left ventricular ejection fraction (assessed by echocardiography and defined as ≥55%) were included. Information on diastolic dysfunction was not collected.
We excluded patients without MACE who were lost to follow-up within the first six months after the transplant. In total, 176 patients being transplanted between the years 2000 and 2015 met these inclusion criteria. In our cohort, only a minority of 4% of the patients (7/176) were transplanted pre-emptively while the vast majority of the patients (96%, 169/176) were on dialysis.
Baseline patient characteristics of this study cohort as well as pre-transplant biomarkers were extracted from medical files and records. Biomarkers were measured before transplant and evaluated retrospectively. The reference range for normal NT-proBNP values was < 125 pg/ml for patients under 75 years of age and < 450 pg/ml for patients older than 75 years.
The calculation of eGFR was based on the Cockroft-Gault equation while body surface area was estimated according to Du Bois.
Outcome assessment
Post-renal transplant major cardiovascular event (MACE) and post-transplant all-cause mortality were the outcomes of interest. MACE was defined as (1) ST-segment elevation myocardial infarction (STEMI), (2) non-ST-segment elevation myocardial infarction (NSTEMI), (3) coronary artery disease needing intervention/bypass surgery, (4) stroke or transient ischemic attack, or (5) mortality due to a cardiovascular cause (defined as death due to coronary artery disease, sudden cardiac death or stroke).
Medical data were used to obtain information on MACE and the cause of death. Follow-up time was defined from the time of kidney transplant until death, loss to follow-up, or the last time their medical records were queried (1st May 2015).
Statistical analysis
For categorical variables, baseline characteristics were presented as absolute number and frequency (percent). For normally distributed, continuous data, results are given as mean and standard deviation (SD). Non-normally distributed, continuous data are presented as median with interquartile range (IQR). Receiver operating characteristic (ROC) curve analysis was performed to assess the capacity of several continuous parameters to predict MACE. When suitable, cut-off values were derived from the point on the ROC curve with a minimum distance to the upper-left corner.
Patients were divided into groups based on their pre-transplant serum NT-proBNP value and groups were compared using Chi-square, Fisher’s exact test, or Student’s t-test, as appropriate.
Univariate Cox regression analysis was applied to identify potential determinants of MACE. The variables derived from univariate analysis were considered for multivariate analysis. Definite variable selection by multivariate Cox regression analysis was then based on the backward elimination of variables with p > 0.05. Fulfillment of the assumption of proportional hazards was graphically assessed by analysis of the Kaplan–Meier curves and the corresponding log-minus-log plots. For the testing for proportional hazards, continuous data were stratified into two groups according to their means or the earlier identified cut-off values. The assumption of proportional hazards was considered as fulfilled when Kaplan–Meier curves did not cross and log-minus-log plots were parallel. All variates considered for and eventually included in the multivariate Cox regression model fulfilled the assumption of proportional hazards. Results of the Cox regression analyses were reported as hazard ratios (HR) with 95% confidence intervals and the corresponding p-value.
The Kaplan–Meier method was used to plot and analyse survival curves and to provide survival rates reported as Kaplan–Meier estimates including a 95% confidence interval in square brackets. A comparison of the survival curves was performed using the log-rank test.
A two-tailed p-value of 0.05 was considered statistically significant. SPSS version 28 was used for statistical analyses.
Results
Characteristics of the cohort
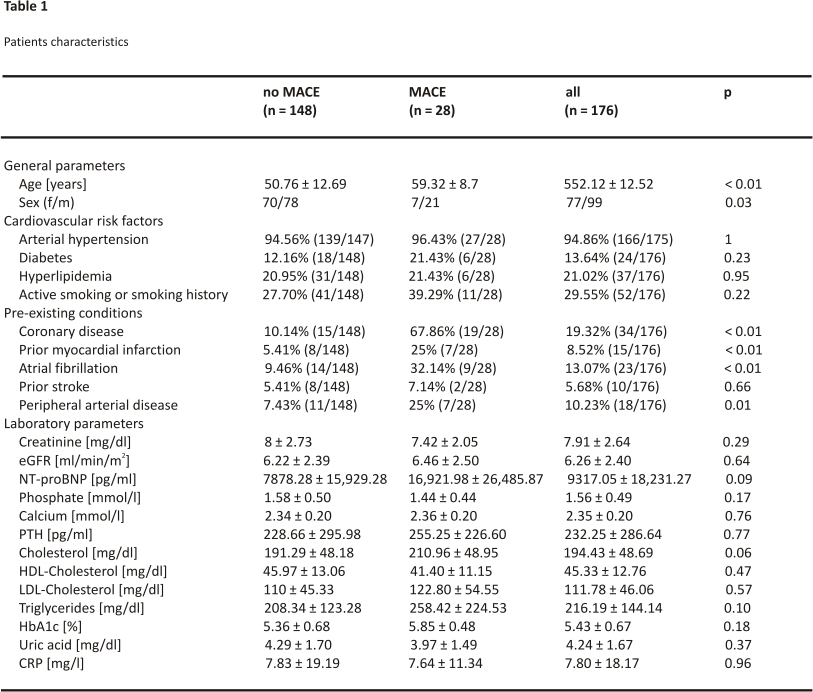
We included 176 patients in our study. Patients were followed up over a median observation time of 38.23 months (IQR: 15.58 to 71.30 months) and the mean age was 52.12 years (± 12.52 years). 56.25% of the cohort was male. In total, MACE occurred in 15.91% of included patients. All of these observed MACE were myocardial infarctions (3 ST-segment elevation myocardial infarctions, 22 non-ST-segment elevation infarctions, and 3 not further specified myocardial infarctions). Patient characteristics as well as pre-existing conditions and laboratory values are given in Table 1.
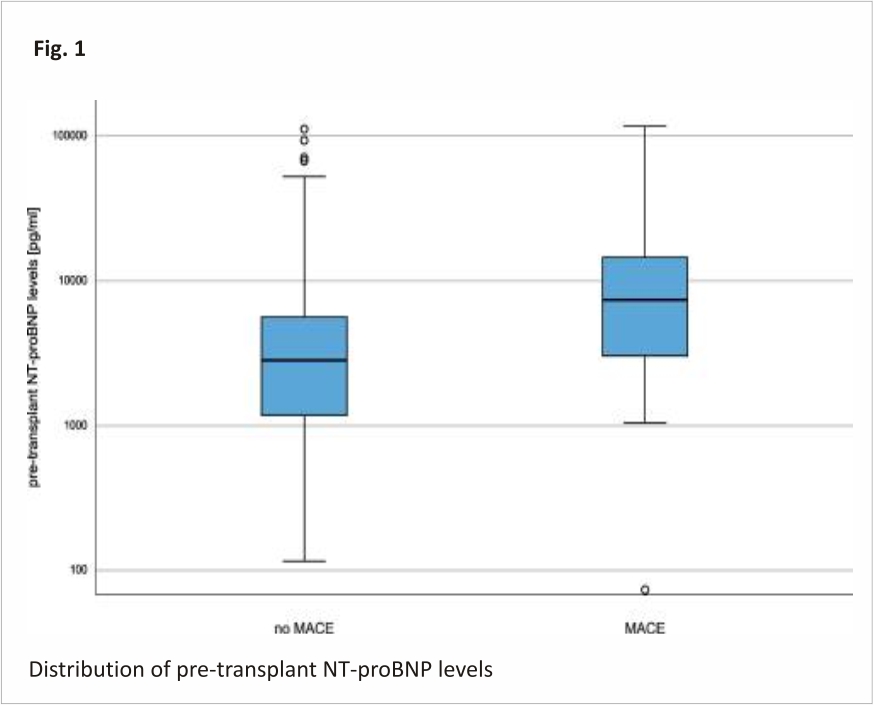
Pre-existing conditions such as coronary artery disease, history of myocardial infarction, atrial fibrillation, and peripheral arterial disease were more common in transplant patients who developed MACE. NT-proBNP showed higher plasma levels in patients with future MACE, but shortly failed statistical significance (NT-proBNP without future MACE: 7878 ± 15,929 pg/ml versus NT-proBNP with future MACE: 16,922 ± 26,486 pg/ml; p = 0.09). Figure 1 visualizes the distribution of NT-proBNP levels for the cohort without vs. with future MACE. Based on eGFR and serum creatinine, kidney function was comparable between those with and without MACE (Table 1).
NT-proBNP levels in patients with and without MACE
The median pre-transplant NT-proBNP level of patients who did not develop post-transplant MACE was 2829.50 pg/ml (IQR: 1173.88 to 5652.50 pg/ml) while patients who developed MACE post-transplant had a median NT-proBNP level of 7452 pg/ml (IQR: 2976.63 to 14,595.90 pg/ml). Note the logarithmic y-axis. Outliners are depicted as circles.
Risk factors for MACE
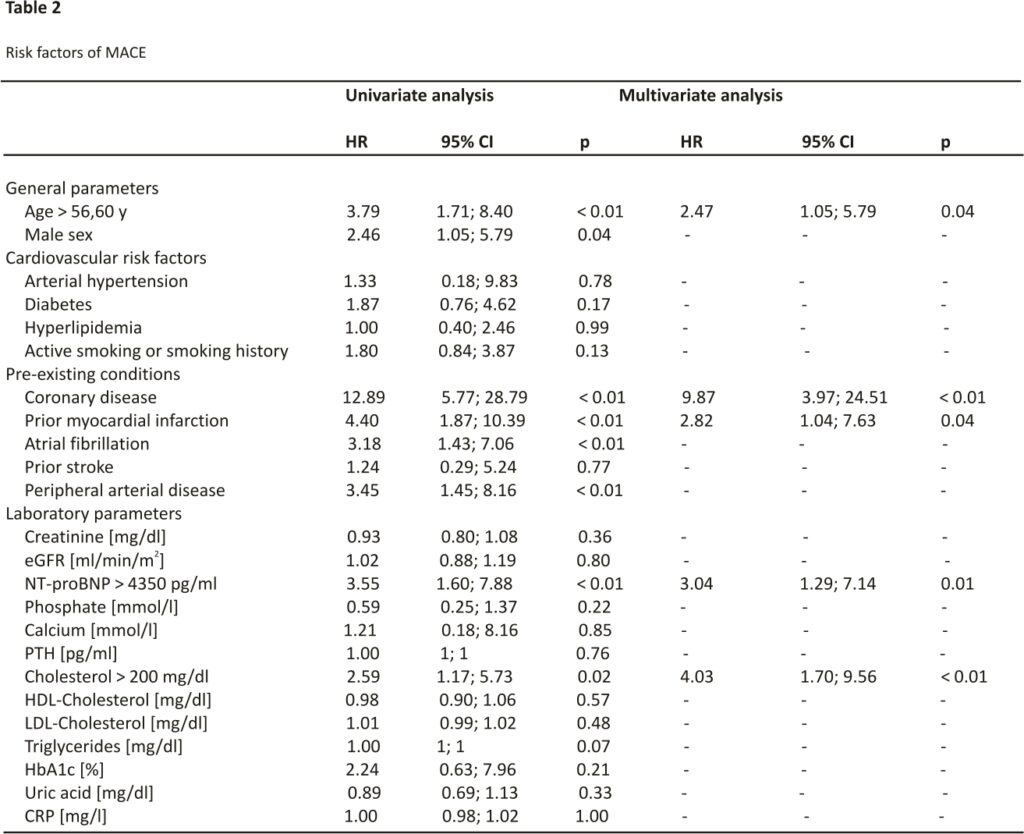
Concerning laboratory markers, no statistically significant differences between the groups with and without MACE could be observed (Table 1). The receiver operating characteristic curve determined the best cut-off value for NT-proBNP to predict MACE at 4350 pg/ml (area under the curve 0.69, sensitivity: 67.86%, specificity: 64.86%, positive predictive value: 26.76%, negative predictive value: 91.43%). Similarly, ROC curve analysis provided suitable cut-off values for the recipient’s age at transplant (56.60 y, area under the curve 0.70, sensitivity: 67.86%, specificity: 66.22%, positive predictive value: 27.54%, negative predictive value: 91.59%) and cholesterol (200 mg/dl, area under the curve 0.62, sensitivity: 61.54%, specificity: 62.77%, positive predictive value: 23.88%, negative predictive value: 89.58%). Pretransplant triglyceride levels did not predict posttransplant MACE.
Univariate analysis identified age, male sex, coronary artery disease, prior myocardial infarction, atrial fibrillation, and peripheral artery disease as risk factors of MACE (Table 2). Furthermore, pre-transplant serum NT-proBNP concentration > 4350 pg/ml was identified as a risk factor for MACE in univariate analysis (Table 2). Interestingly, serum creatinine as well as creatinine-based eGFR did not associate with MACE.
In multivariate analysis, NT-proBNP above 4350 pg/ml was confirmed as an independent risk factor for MACE (HR 3.04; p = 0.01). Besides NT-proBNP, also recipient’s age at transplant > 56.60 years (HR 2.47; p = 0.04), cholesterol above 200 mg/dl (HR 4.03; p < 0.01), coronary artery disease (HR 9.87; p < 0.01) and history of myocardial infarction (HR 2.82; p = 0.04) (Table2) could be identified as risk factors for MACE.
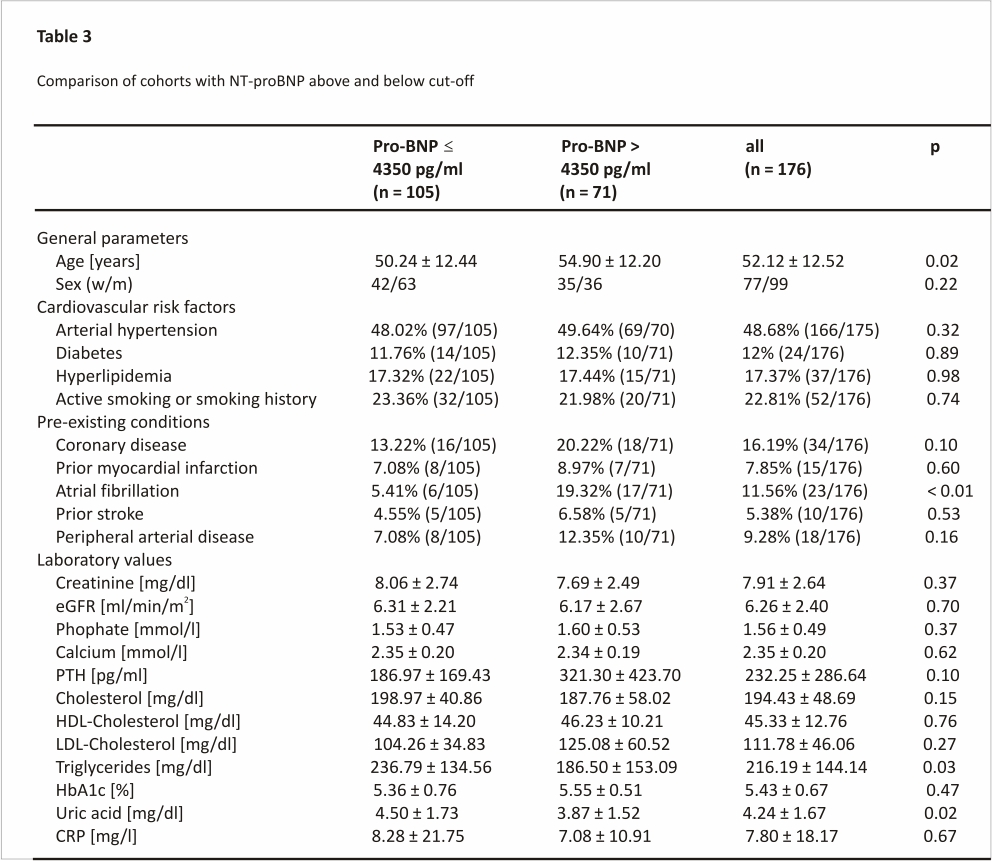
To better characterize patients with high NT-proBNP levels, we compared patients with a pre-transplant NT-proBNP level > 4350 pg/ml to those below this threshold. Patients with NT-proBNP levels above the identified cut-off were of older age, suffered more often from atrial fibrillation and had lower triglyceride and uric acid level (Table 3).
NT-proBNP and survival
Patients developing MACE over time showed a 1- and 5-year survival rate of 73.95% [62.70%; 85.20%] and 25.88% [13.40%; 38.37%], respectively. 1- and 5-year survival rates for patients without MACE were 98.65% [97.70%; 99.60%] and 88.25% [84.98%; 91.51%] (log-rank test: p < 0.01, see Fig. 2A).
To analyse if our identified NT-proBNP cut-off discriminates between patients at higher risk of death, we plotted Kaplan–Meier curves for patients with NT-proBNP below and above our threshold of 4350 pg /ml. Patients with NT-proBNP levels above 4350 pg/ml had 1- and 5-year survival rates of 90.67% [87.04%; 94.30%] and 68.20% [61,38%; 75.03%], whereas patients with NT-proBNP levels below 4350 pg/ ml had 1- and 5-year survival rates of 100% and 90.48% [86.68%; 94.27%] (log-rank test: p < 0.01, see Fig. 2B).
To further address this finding, we stratified the patients according to their NT-proBNP levels in four groups based on the earlier identified cut-off value. NT-proBNP levels inversely correlated with survival (pooled log-rank test: p < 0.01, see Fig. 2C).
NT-proBNP and MACE-free survival
Figure 3 illustrates the MACE-free survival of all patients irrespective of their pre-transplant serum NT-proBNP levels. To analyze whether or not serum NT-proBNP levels correlate with MACE-free survival, we again plotted Kaplan– Meier curves for patients with NT-proBNP below and above the identified cut-off value of 4350 pg/ml (Fig. 3B). 1- and 5-year MACE-free survival rates were calculated as 93.33% [90.90%; 95.77%] and 91.21% [88.04%; 94.38%] for patients with NT-proBNP < 4350 pg/ml and 78.82% [73.97%; 83.68%] and 74.68% [69.26%; 80.10%] for patients with NT-proBNP > 4350 pg/ml (log-rank test for the Kaplan–Meier curves: p < 0.01). Again, we further stratified the patients according to their NT-proBNP levels in four groups (Fig. 3C). The corresponding Kaplan–Meier curves differed significantly (pooled log-rank test: p = 0.01) and MACE-free survival inversely correlated with NT-proBNP levels.
1- and 5-year MACE-free survival rates were the highest in patients with NT-proBNP ≤2175 pg/ml (both 93.94% [91%; 96,88%]) and the lowest in patients with NT-proBNP ≥6525 pg/ml (78.20% [72.10%; 84.30%] and 71.54% [64.34%; 78.74%], respectively). Patients with NT-proBNP between 2175 pg/ml and 4350 pg/ml (1- and 5-year MACE-free survival rates: 92.31% [88.04%; 96.57%] and 85.71% [78.23%; 93.20%], respectively) and between 4350 and 6525 pg/ml (1- and 5-year MACE-free survival rates: both 80% [72%; 88%]) showed intermediate MACE-free survival rates compared to the groups of low and high NT-proBNP (Fig. 3C).
Discussion
Pre-transplant systolic dysfunction is associated with mortality after transplantation 17. But even within the group of patients with preserved left ventricular ejection fraction, significant differences concerning the risk for future cardiovascular events exist. Therefore, biomarkers at the time of transplantation may identify patients at greater risk for cardiovascular complications and death. In a recent study, NT-proBNP, renal function, and the need for anti-hypertensive medication have been identified as stronger risk factors for mortality in a cohort of renal transplant recipients in comparison to the general population 13, but all laboratory parameters have been measured at various time points after transplantation. In our study aiming at the identification of a predictive factor, NT-proBNP was measured shortly before transplantation.
In our cohort of 176 adult renal transplant recipients with preserved left ventricular ejection fraction, we demonstrated a significant association between pre-transplant NT-proBNP and post-transplant MACE and confirmed NT-proBNP as an independent risk factor for MACE and survival. Our findings are in line with previous studies, which showed that greater elevations of NT-proBNP were associated with a higher risk for cardiovascular mortality 3. Since NT-proBNP is released upon ventricle stretch as well as myocardial damage and is at least partially cleared by the kidneys, it is usually measured above thresholds in ESKD. This raises two main questions. Firstly, a new biomarker stratification is needed especially for wait-listed patients, because the common thresholds do not apply in these patients. Secondly, how to interpret pre-transplant NT-proBNP results in patients after renal transplantation.
Here we could identify a pre-transplant NT-proBNP cut-off of 4350 pg/ml to predict MACE-free survival as well as overall survival in patients after renal transplantation. Therefore, NT-proBNP might help to identify renal transplant recipients at greater risk for cardiovascular mortality.
We further suggest that NT-proBNP as a widely available biomarker can be used for the identification of patients at cardiovascular risk pre-transplantation regardless of their medical history or preexisting coronary artery disease. In our cohort, one-third of patients with a typical history did not develop MACE after kidney transplant, so NT-proBNP measurement may help to stratify patients better. However, our identified cut-off value has to be validated in a larger cohort and a prospective study design.
The relatively low overall NT-proBNP value is most likely due to the inclusion of patients with preserved left ventricular ejection fraction. For patients with heart failure and reduced ejection fraction (HfrEF), NT-proBNP values are much higher so NT-proBNP is an established diagnostic and prognostic marker of congestive heart failure and left ventricular systolic dysfunction 18. Therefore, it is essential the use NT-proBNP as a prognostic marker to define appropriate subgroups according to the heart function. Within our study, we do not have information on diastolic dysfunction or signs of cardiac hypertrophy leading to increased NT-proBNP levels. However, since BNP was not closely related to diastolic dysfunction in a population-based study, the presence of diastolic dysfunction would not be expected to bias our results considerably 19. To date, there is a lack of published data regarding the relationship between NT-proBNP concentrations and diastolic dysfunction in ESKD patients 20. In conclusion, we could demonstrate that especially in our cohort of relatively healthy patients with preserved left ventricular ejection fraction, pre-transplant NT-proBNP is a valuable tool to identify transplant recipients at high cardiovascular risk.
We found that extreme NT-proBNP values classified patients better than intermediate values. In studies comprising an even larger number of patients, these less marked differences may become more prominent.
Although we cannot provide longitudinal data, analysis of NT-proBNP trajectory after renal transplantation might be of great interest. As demonstrated previously, an association between change in NT-proBNP at various time points and mortality is well established 21–23. This effect was not limited to cohorts of dialysis patients. Also in a cohort of patients with CKD 3–4, patients with the greatest change in NT-proBNP had the poorest survival 24.
In our cohort, serum creatinine, as well as creatinine-based eGFR, did not show any difference between the groups of patients with and without MACE. Creatinine-based eGFR is of limited accuracy in describing residual renal function in ESKD patients. Interestingly, it has been shown that NT-proBNP had more influence on the difference in mortality between renal transplant recipients and the general population than creatinine clearance 13.
This is in line with a most recent published study of our group 25. Pre-transplant residual renal function assessed by serum creatinine and serum cystatin C values were not significantly associated with MACE, whereas another biomarker of kidney function Beta-Trace protein (BTP) was. BTP has been demonstrated to be tightly associated with cardiovascular risk 26, which might explain these findings. In the cohort analyzed here, BTP was not associated with cardiovascular risk (data not shown), most likely because only patients with preserved left ventricular ejection fraction were included.
Also, the limitations of our study should be mentioned. Due to the observational design of our study, we cannot draw conclusions about the causal associations between pre-transplant NT-proBNP levels and post-transplant MACE as well as survival. Blood was taken immediately before transplantation, however, we cannot rule out moderate differences with regard to excess volume depending on the last dialysis treatment, which might have taken place from 0 up to 72 h before transplantation. All patients were treated to the standard of care, so that an imbalance in cardioprotective medication between the NT-proBNP groups, which was not related to the previous diagnosis of cardiovascular disease, is unlikely. However, no prescribing information is available.
In conclusion, pre-transplant serum NT-proBNP as a widely available biomarker is associated with post-transplant MACE as well as survival. Interpretation of pre-transplant NT-proBNP might help to identify patients at high risk for an undesired outcome.
Acknowledgments
Not applicable.
Authors’ contributions
SS: analysis and interpretation of data, drafting of the manuscript. DP: analysis and interpretation of data. CEK: acquisition, analysis, and interpretation of data, RW: acquisition and analysis of data, LW: acquisition and analysis of data, SR: acquisition and analysis of data, DB: acquisition and analysis of data, CS: analysis and interpretation of data, reviewing the manuscript, SvV: analysis and interpretation of data, reviewing the manuscript, PL: analysis and interpretation of data, drafting of the manuscript, RPW: study concept and design, interpretation of data, administrative support, study supervision. The author(s) read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study and waiver of the informed consent were approved by the local ethic committee (Ethics Committee of the Medical Faculty, University Hospital Bonn, University of Bonn, Building 74/4th floor, Venusberg-Campus 1, 53105 Bonn, Germany, Number of ethics committees statement concerning this study: 159/17). The study was conducted according to the principles of the Declaration of Helsinki.
We confirm that no organs were obtained from prisoners. Deceased donor organs were allocated by Eurotransplant. Living donor kidney transplantation was conducted at the university hospital of Bonn.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Philipp Lutz and Rainer P. Woitas contributed equally to this work.
References
1. Wolfe RAV, Milforf E, Ojo A, Ettenger R, Agodoa L, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341 (23):1725–1730. doi: 10.1056/NEJM 199912023412 303.
2. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496– 506. doi:10.1681/asn.2004070580.
3. Harrison TG, Shukalek CB, Hemmelgarn BR, Zarnke KB, Ronksley PE, Iragorri N, et al. Association of NT-proBNP and BNP with future clinical outcomes in patients With ESKD: a systematic review and meta-analysis. Am J Kidney Dis. 2020;76(2):233–247. doi: 10.1053 /j.ajkd.2019.12.017.
4. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46(4):610–620. doi:10.10 53/j.ajkd.2005.06.017.
5. Gangji AS, Helal BA, Churchill DN, Brimble KS, Margetts PJ. Association between n-terminal propeptide b-type natriuretic peptide and markers of hypervolemia. Perit Dial Int. 2008; 28(3):308–311. doi: 10.1177/089686 080802800319.
6. Niizuma S, Iwanaga Y, Yahata T, Miyazaki S. Renocardiovascular biomarkers: from the perspective of managing chronic kidney disease and cardiovascular disease. Front Cardiovasc Med. 2017;4. 10.3389/ fcvm.2017.00010.
7. Jha V, Sundqvist S, Larson T, Cauliez B, Bauer F, Dumont A, et al. Clinical value of natriuretic peptides in predicting time to dialysis in stage 4 and 5 chronic kidney disease patients. Plos One. 2016;11(8) :e0159914. doi:10.1371/journal. pone.0159914.
8. Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care. 2012;16 (3): R74. doi:10.1186/cc11331.
9. Herzog CA, Pearce LA, Murakami MM, Apple FS. Multi-biomarker risk stratification of n-terminal pro-b-type natriuretic peptide, high-sensitivity c-reactive protein, and cardiac troponin t and I in end-stage renal disease for all-cause death. Clin Chem. 2004;50 (12):2279– 2285. doi: 10.1373/clinchem.2004. 035741.
10. Srisawasdi P, Jacobs LH, Mingels AM, Wodzig WK, van Dieijen-Visser MP, Kooman JP, et al. Renal dysfunction, hemodialysis, and the NT-proBNP/ BNP ratio the authors’ reply. Am J Clin Pathol. 2010;134(3):516–517. doi: 10.1309/ajcpizhtdsr2oggx.
11. Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement. J Am Coll Cardiol. 2006;47(1):91–97. doi: 10.1016/ j.jacc.2005.08.051.
12. Wang AY-M, Lai K-N. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol. 2008;19(9):1643 –52. doi:10.1681/asn.200 801001 2.
13. Oterdoom LH, de Vries APJ, van Ree RM, Gansevoort RT, van Son WJ, van der Heide JJH, et al. N-terminal pro-b-type natriuretic peptide and mortality in renal transplant recipients versus the general population. Trans plantation. 2009;87(10):1562–1570. doi:10.1097/TP.0b013e3181a4bb80.
14. Heringlake M, Heide C, Bahlmann L, Eichler W, Pagel H, Schmucker P, et al. Effects of tilting and volume loading on plasma levels and urinary excretion of relaxin, NT proANP, and NT proBNP in male volunteers. J Appl Physiol. 2004; 97:113–179. doi: 10.1152/jappl physiol.01196.2003.
15. Schou M, Dalsgaard MK, Clemmesen O, Dawson EA, Yoshiga CC, Nielsen HB, et al. Kidneys extract BNP and NT-proBNP in healthy young men. J Appl Physiol. 2005;99(5):1676 –1680. doi: 10.1152/japplphysiol .00641.2005.
16. Hausken J, Klingenberg O, Morkrid L, Rollag H, Foss A, Midtvedt K. Elevated troponin I and NT-proBNP at the time of transplantation may predict a major adverse cardiac event in the early postoperative period after renal transplantation. Transplantation. 2012;94(2):e13– e15. doi:10.1097/TP.0b013e318 25b75df.
17. Siedlecki A, Foushee M, Curtis JJ, Gaston RS, Perry G, Iskandrian AE, et al. The impact of left ventricular systolic dysfunction on survival after renal transplantation. Trans plantation. 2007;84(12):1610– 1617. doi:10. 1097/01.tp.0000 295748.42884.97.
18. McCullough PA, Duc P, Omland T, McCord J, Nowak RM, Hollander JE, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the breathing not properly multinational study. Am J Kidney Dis. 2003;41(3): 571–579. doi: 10.1053/ajkd.2003. 50118.
19. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction. Circulation. 2004;109(25):3176 –3181. doi: 10.1161/01.cir.0000130845.381 33.8f.
20. Memon L, Spasojevic-Kalimanovska V, Stanojevic NB, Kotur-Stevuljevic J, Simic-Ogrizovic S, Giga V, et al. Are levels of NT-proBNP and SDMA useful to determine diastolic dysfunction in chronic kidney disease and renal transplant patients? J Clin Lab Anal. 2013;27(6):461–470. doi: 10.1002/ jcla.21628.
21. Leuchte HH, Baumgartner RA, Nounou ME, Vogeser M, Neurohr C, Trautnitz M, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med. 2006;173(7): 744–750. doi: 10.1164/rccm.200 510-1545OC.
22. Mallamaci F, Zoccali C, Tripepi G, Benedetto FA, Parlongo S, Cataliotti A, et al. Diagnostic potential of cardiac natriuretic peptides in dialyses patients. Kidney International. 2001; 59:1559–1566. doi: 10.1046/j.1523-1755.2001. 0590041559.x.
23. Nakatni T, Naganuma T, Masuda X, Uchida J, Suimura T, Sugimura K. Significance of brain natriuretic peptides in patients on continuous ambulatory peritoneal dialysis. Int J Mol Med. 2002;10:457–461.
24. Roberts MA, Srivastava PM, Macmillan N, Hare DL, Ratnaike S, Sikaris K, et al. B-type natriuretic peptides strongly predict mortality in patients who are treated with long-term dialysis. Clin J Am Soc Nephrol. 2008; 3(4):1057– 1065. doi:10.2215/cjn. 05151107.
25. Schwab S, Pörner D, Kleine CE, Werberich R, Werberich L, Reinhard S, et al. Pre-transplant serum beta trace protein indicates risk for post-transplant major cardiac adverse events. Nephrology. 2022 doi: 10.1111/nep.14131.
26. White CA, Ghazan-Shahi S, Adams MA. beta-Trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis. 2015;65(1):1 31–146. doi: 10.1053/j.ajkd.2014. 06.038.
CREDITS: Schwab S, Pörner D, Kleine CE, Werberich R, Werberich L, Reinhard S, Bös D, Strassburg CP, von Vietinghoff S, Lutz P, Woitas RP. NT-proBNP as a predictor of major cardiac events after renal transplantation in patients with preserved left ventricular ejection fraction. BMC Nephrol. 2023 Feb 11;24(1):32. doi:10.1186/s12882-023-03082-9. PMID: 36774457; PMCID: PMC9922448.