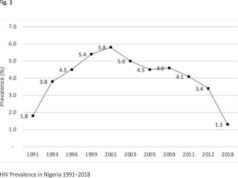
Pragya D Yadav, PhD, Gajanan N Sapkal, PhD, Raches Ella, MS, Rima R Sahay, PhD, Dimpal A Nyayanit, PhD, Deepak Y Patil, PhD, Gururaj Deshpande, PhD, Anita M Shete, PhD, Nivedita Gupta, MD, PhD, V Krishna Mohan, PhD, Priya Abraham, MD, PhD, Samiran Panda, MD, PhD, Balram Bhargava, DM
To whom correspondence should be addressed.
Pragya D Yadav, PhD – Indian Council of Medical Research-National Institute of Virology, Pune, India Tel: +9120-26006111; Fax: 91-20-26122669; Email: hellopragya22@gmail.com
Keywords: SARS-CoV-2, Beta, Delta, vaccine efficacy, neutralization
Topic: vaccines serum neutralization sars-cov-2 covid-19 covid-19 vaccines
Issue Section: RAPID COMMUNICATION
In the last several months, several SARS-CoV-2 variants have emerged from various countries worldwide.1 Among them, a variant of concern (VOC) i.e. Alpha (B.1.1.7), Beta (B.1.351), Gamma (B.1.1.28.1) and Delta (B.1.617.2) are serious public health threats because of their association with the higher transmissibility and the potential immune escape.1,2 Various reports have been published on the neutralization efficacies with the sera of the currently available COVID-19 vaccines against these variants.1,2 However, the immune escape of the Beta variant has been a serious concern for the COVID-19 vaccination programme. It has shown reduced neutralization to several approved vaccines such as mRNA-1273, BNT162b2, ChAdOx1 nCoV-19, NVX-CoV2373.1 Another reason for global concern is the recent emergence and detection of highly transmissible Delta variants from India and various other countries.2 An inactivated SARS-CoV-2 vaccine, BBV152/Covaxin was rolled out under the national COVID-19 vaccination programme in India. The neutralization potential of the BBV152 has been already studied with the B.1, Alpha, Zeta and Kappa found to be effective against these variants.3–5
Here, we assessed the neutralization of sera from COVID-19 recovered cases (n = 20) post 5– 20 weeks of infection and vaccines 28 days after two doses of BBV152 (n = 17) against Beta, Delta variants and compared with prototype B.1 (D614G). The recovered cases were infected with B.1 (n = 17) and B.1.617.1 lineage (n = 3). SARS-CoV-2 isolates were propagated at the Indian Council of Medical Research-National Institute of Virology (ICMR-NIV), Pune from the clinical samples using Vero CCL-81 cells and were used for a 50% plaque reduction neutralization test (PRNT50).
Geometric mean titre (GMT) for vaccinees sera against B.1, Beta and Delta variants were found to be 187.5 [95% confidence interval (CI): 129.3–271.9], 61.57 (95%CI: 36.34– 104.3) and 68.97 (95%CI: 24.72– 192.4), respectively. The GMT ratio of B.1 to Beta and Delta variants was 3.0 (95%CI: 2.6–3.6) and 2.7 (95% CI: 1.4–5.2).
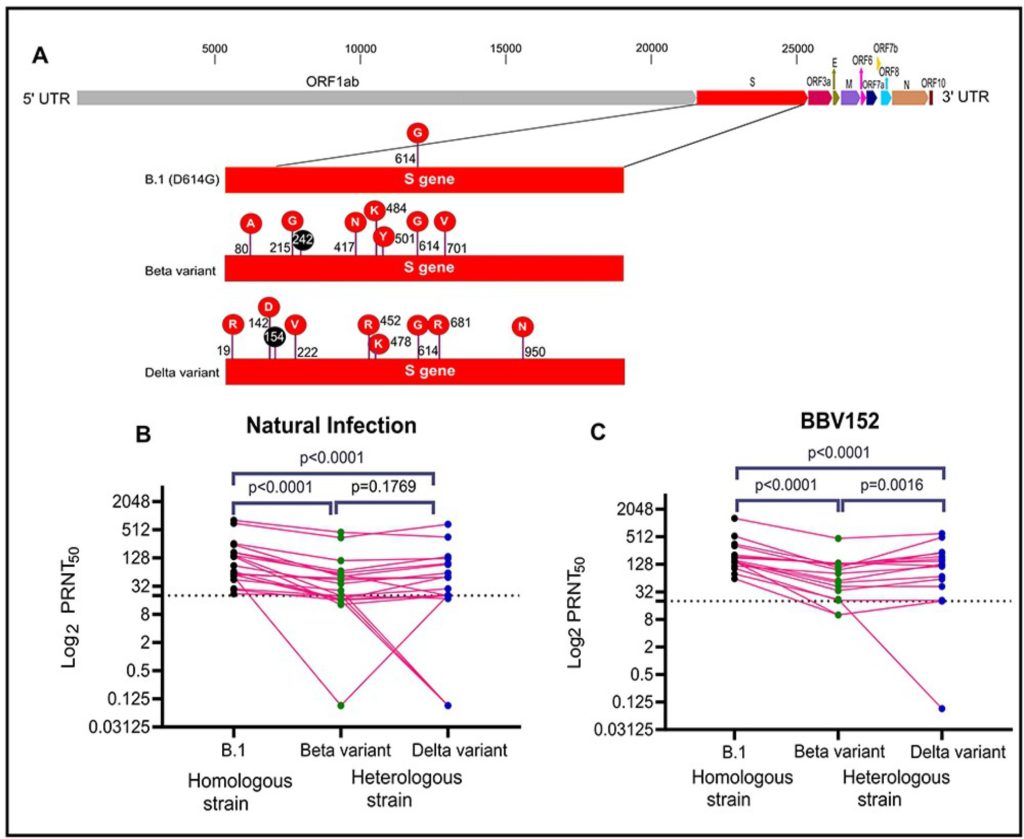
Similarly, GMT titers in sera of recovered cases against B.1, Beta and Delta variants were 97.8 (95%CI: 61.2– 156.2), 29.6 (95%CI: 13.4–65.0) and 21.2 (95% CI: 6.4–70.1), respectively. The GMT ratio of B.1 to Beta and Delta variants was 3.3 (95%CI: 2.4–4.5) and 4.6 (95% CI: 2.2– 9.5). Sera of vaccinees and recovered cases had shown a significant reduction in neutralization titre for Beta and Delta variants in comparison to B.1 (P-value: < 0.0001) (Figure 1).
Several studies have reported the reduction in the neutralization efficacy with the sera of naturally infected cases and individuals vaccinated with BBIBP-CorV (1.6×), BNT162b2 (6.5×), mRNA-1273 (8.6×), ChAdOx1 nCoV-19 (86×) against Beta variant.1,6 Reduced neutralization with the vaccinees’ sera of BNT162b2 mRNA (7×) and one dose of ChAdOx1 nCoV-19 was observed against Delta.2
Our study demonstrated that despite a reduction in neutralization titers with BBV152 vaccinees sera against Beta and Delta variants, its neutralization potential is well established. Lastly, the broad epitope coverage in an inactivated vaccine induces an immune response against the whole virion, which decreases the magnitude of reduced neutralization against emerging variants. Further mutation of Delta variant known as ‘Delta AY.1 and Delta AY.2’ has been identified from India and other countries, which has been a threat to existing vaccines and drugs. We still do not know how reduced neutralization activity results in reduced vaccine effectiveness; hence, vaccine effectiveness studies are absolutely required to fully appreciate the effectiveness of BBV152 against these two variants.7
Ethical Approval
The study was approved by the Institutional Biosafety Committee and Institutional Human Ethics Committee of ICMR-NIV, Pune, India under the project ‘Comparative assessment of BBV152 vaccine (COVAXIN™) antibody and antigen-specific responses in immunized population without past COVID-19 infection, individuals vaccinated after recovery from COVID-19 and non-vaccinated individuals with past COVID-19 infection’ (21-2-6N).
Conflicts of Interest:
R.E. and V.K.M are employees of Bharat Biotech International Limited, Hyderabad, with no stock options or incentives. P.D.Y., G.N.S., R.R.S., D.A.N., D.Y.P., G.D., A.M.S., P.A., N.G., S.P. and B.B. are the employees of the Indian Council of Medical Research, New Delhi and they do not have any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Author Contributions
P.D.Y., R.E. and P.A. contributed to study design, data collection, data analysis, interpretation and writing and critical review. G.N.S., R.R.S., D.A.N., D.Y.P., G.D. and A.M.S. contributed to data collection, interpretation, writing and critical review. N.G., S.P., V.K.M and B.B. contributed to the critical review and finalization of the paper.
Acknowledgements
Authors gratefully acknowledge the staff of ICMR-NIV, Pune including Mr Prasad Sarkale, Mr Shreekant Baradkar, Ms Aasha Salunkhe and Mr Chetan Patil for extending excellent technical support.
Funding
The Indian Council of Medical Research (ICMR), New Delhi provided funding to ICMR-National Institute of Virology, Pune under intramural funding ‘COVID-19’.
References
1. Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants —clinical, public health, and vaccine implications. N Engl J Med 2021; 384:1866–8. 10.1056/ NEJMc2100362.
2. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B. 1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv. 2021. 05.26.445838, doi 10.1101/ 2021.05.26.445838, preprint: not peer-reviewed.
3. Sapkal GN, Yadav P, Ella R et al. Inactivated COVID-19 vaccine BBV152/ COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Travel Med 2021; 28:taab051.
4. Sapkal G, Yadav PD, Ella R et al. Neutralization of B. 1.1. 28 P2 variants with sera of natural SARS-CoV-2 infection and recipients of inactivated COVID-19 vaccine Covaxin. J Travel Med 2021; taab077.
5. Yadav P, Sapkal GN, Abraham P et al. Neutralization of variant under investigation B. 1.617 with sera of BBV152 vaccinees. Clin Infect Dis 2021; ciab411. 10.1093/cid/ciab411.
6. Wibmer CK, Ayres F, Hermanus T et al. SARS-CoV-2 501Y. V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021; 27:622–5.
7. Patel MK, Bergeri I, Bresee JS et al. Evaluation of post-introduction COVID-19 Vaccine Effectiveness: Summary of interim guidance of the World Health Organization. Vaccine 2021; 39:4013 –24.
Credit: Pragya D Yadav, PhD, Gajanan N Sapkal, PhD, Raches Ella, MS, Rima R Sahay, PhD, Dimpal A Nyayanit, PhD, Deepak Y Patil, PhD, Gururaj Deshpande, PhD, Anita M Shete, PhD, Nivedita Gupta, MD, PhD, V Krishna Mohan, PhD, Priya Abraham, MD, PhD, Samiran Panda, MD, PhD, Balram Bhargava, DM, Neutralization of Beta and Delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152/Covaxin, Journal of Travel Medicine, 2021; taab104, https:// doi.org/10.1093/jtm/taab104