Daniel B. Rosoff, Andrew S. Bell, Jeesun Jung, Josephin Wagner, Lucas A. Mavromatis, and Falk W. Lohoff
Abstract
Background
Lipid-lowering therapy with statins and proprotein convertase subtilisin/Kexin type 9 (PCSK9) inhibition are effective strategies in reducing cardiovascular disease risk; however, concerns remain about potential long-term adverse neurocognitive effects.
Objectives
This genetics-based study aimed to evaluate the relationships between long-term PCSK9 inhibition and statin use on neurocognitive outcomes.
Methods
We extracted single-nucleotide polymorphisms in 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) and PCSK9 from predominantly European ancestry-based genome-wide association studies summary-level statistics of low-density lipoprotein cholesterol and performed drug-target Mendelian randomization, proxying the potential neurocognitive impact of drug-based PCSK9 and HMGCR inhibition using a range of outcomes to capture the complex facets of cognition and dementia.
Results
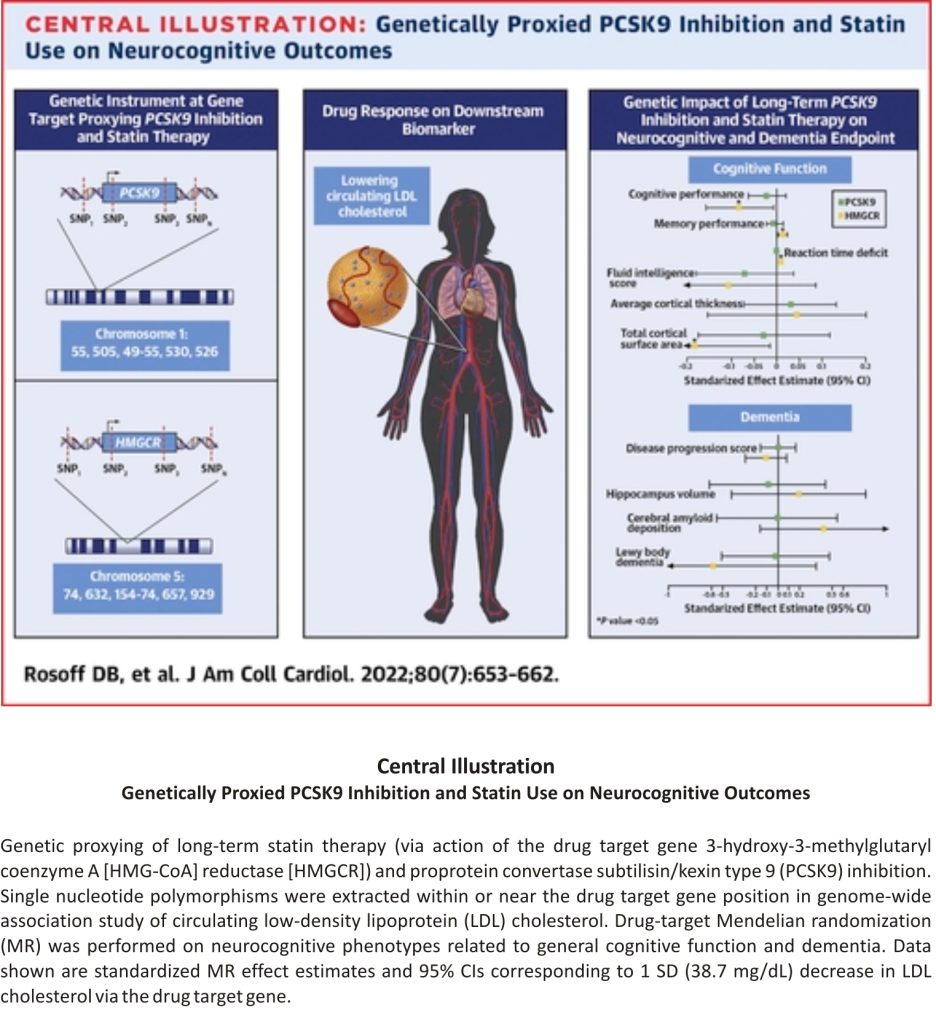
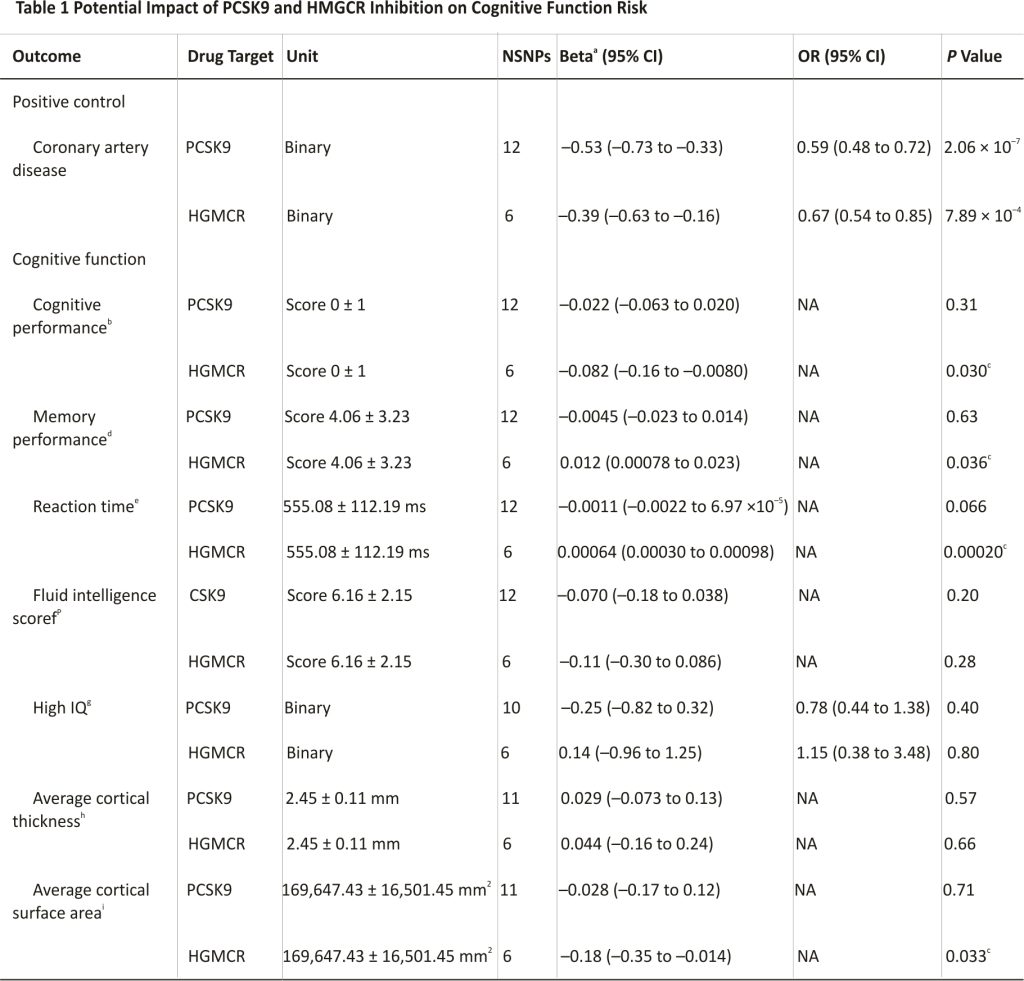
Using data from a combined sample of ∼740,000 participants, we observed a neutral cognitive profile related to genetic PCSK9 inhibition, with no significant effects on cognitive performance, memory performance, or cortical surface area. Conversely, we observed several adverse associations for HMGCR inhibition with lowered cognitive performance (beta: –0.082; 95% CI: –0.16 to –0.0080; P = 0.03), reaction time (beta = 0.00064; 95% CI: 0.00030-0.00098; P = 0.0002), and cortical surface area (beta = –0.18; 95% CI: –0.35 to –0.014; P = 0.03). Neither PCSK9 nor HMGCR inhibition impacted Alzheimer’s disease progression biomarkers or Lewy body dementia risk. Consistency of findings across Mendelian randomization methods accommodating different assumptions about genetic pleiotropy strengthens causal inference.
Conclusions
Using a wide range of cognitive function and dementia endpoints, we failed to find genetic evidence of an adverse PCSK9-related impact, suggesting a neutral cognitive profile. In contrast, we observed adverse neurocognitive effects related to HMGCR inhibition, which may well be outweighed by the cardiovascular benefits of statin use but may warrant pharmaco- vigilance.
Introduction
Cardiovascular disease remains the leading cause of mortality and morbidity with an estimated annual 17.9 million deaths worldwide.1 Although lifestyle modifications can reduce cardiovascular disease risk, pharmacotherapeutic strategies are important tools for managing cardiovascular health in high-risk patients.2,3 Statins are the standard of care for hypercholesterolemia.4,5 Despite their widespread use in hypercholesterolemic patients, they may still be under-used overall in part due to suspected adverse side effects, including impaired cognition and increased dementia risk.6-10 Although recent observational studies have reported that statins are not associated with cognitive decline, case studies have found cognitive and memory impairment in patients taking statins.11,12 By contrast, other studies have suggested that statins may decrease the risk of dementia, and Alzheimer’s disease (AD), and reduce cognitive impairment in certain patients, which together highlights the continued uncertainty regarding the neurocognitive impact of statins.13,14
Proprotein convertase subtilisin/Kexin type 9 (PCSK9), an important regulator of low-density lipoprotein cholesterol (LDL-C), has emerged as another target for cholesterol-lowering drug development.15-17 The first class of PCSK9 inhibitor (PCSK9i) drugs include the monoclonal antibodies alirocumab and evolocumab, which have been extensively studied for a range of cardiovascular outcomes in several phases II and III clinical trials and are U.S. Food and Drug Administration approved to treat adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who require additional lowering of LDL-C.18-21 In addition, the first RNA interference PCSK9 drug, inclisiran, has been recently approved by the European Commission and the U.S. Food and Drug Administration.22-25 However, the long-term effects of PCSK9 inhibition on neurocognitive outcomes have not been studied.
Short-term randomized control trial data suggest PCSK9 inhibition has a safe neurocognitive side-effect profile.20,26-28 The EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) trial, which evaluated a subset of the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects with Elevated Risk) phase III evolocumab clinical trial, followed a large group of patients administered evolocumab and reported no significant adverse neurocognitive outcomes.27,29 However, these patients were already on stable moderate- to high-intensity statin therapy, which complicates disentangling drug-specific effects on neurocognition.20,27 PCSK9 is known to affect central nervous system development, neurodifferentiation, and neuronal apoptosis; thus, inhibiting PCSK9 may impact brain functions.30-32 Moreover, neural PCSK9 expression is upregulated in adult brains during disease states, including AD, alcohol use disorder, ischemic stroke, and neuropsychiatric disorders.32 Thus, given the paucity of long-term randomized controlled trial data evaluating the impact of PCSK9 inhibition on neurocognition, preclinical studies highlighting a potential role of PCSK9 in brain function, and continued development of additional PCSK9 inhibitors, it is important to determine whether long-term PCSK9 inhibition is associated with neurocognitive side effects.
Drug-target Mendelian randomization (MR) analyzes genetic variants that simulate the pharmacological inhibition of drug-gene targets with regression estimates reflecting the impact of long-term drug use and is an important analytical tool to strengthen causal inference regarding the potential impact of these drug target genes in cardiovascular disease, cognition, and dementia.33-36 However, the underlying neurobiology of both cognition and dementia is complex, and previous MR studies only included broad neurocognitive variables. Therefore, a finer-grained analysis using detailed neurocognitive outcomes would help to identify any adverse effects that may limit the clinical benefits of PCSK9i drugs and would facilitate comparisons between this new intervention and standard-of-care statins.
In this study, we use recently released genome-wide association study (GWAS) data and drug-target MR to examine the potential impact of PCSK9 and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) inhibition across several cognitive-related outcomes aimed to capture potential distinct cognition-related relationships with PCSK9 and HMGCR inhibition, such as cognitive performance, memory performance, and reaction time. We also use structural neuroimaging data, biomarkers of AD, and Lewy body dementia (LBD) risk to complement and extend the previous drug-target MR studies by investigating biologically informed markers of dementia.
Methods
Approval and data sources
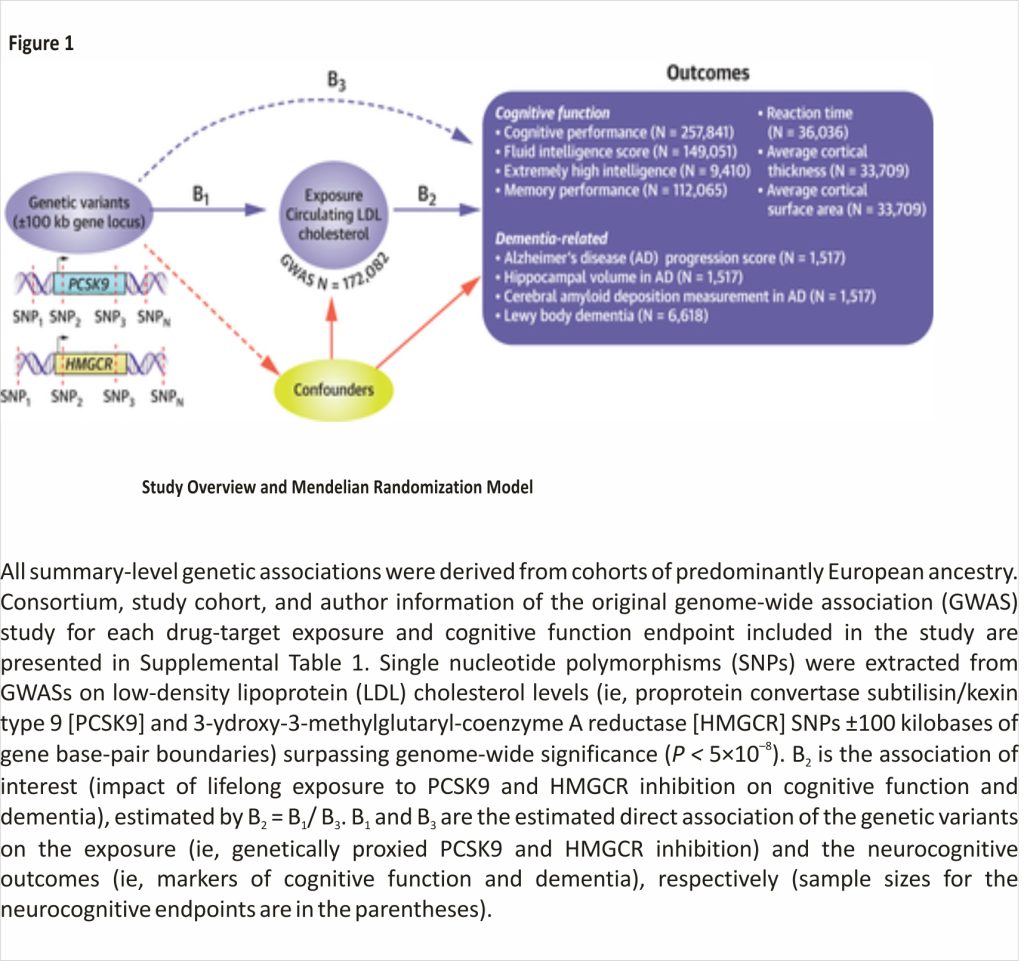
This study uses publicly available, summary-level GWAS data (Figure 1). All data sources used (Alzheimer’s Disease Neuroimaging Initiative [ADNI]37; UK Biobank [UKB]38; Global Lipid Genomics Consortium [GLGC]39; Enhancing Neuroimaging Genetics through Meta-Analysis [ENIGMA]40; and CARDIoGRAMplusC4D [Coronary Artery Disease Genome-Wide Replication and Meta-analysis (CARDIoGRAM) plus The Coronary Artery Disease (C4D) Genetics]41) have existing approvals from their respective Institutional Review Boards. All participants provided written informed consent. Full information and references for data sources are in Supplemental Table 1.
PCSK9 and HMGCR genetic instrument selection
To proxy therapeutic inhibition of PCSK9 and HMGCR, we used single-nucleotide polymorphisms located within 100 kilobases of PCSK9 and HMGCR and associated with lower LDL-C from GLGC GWAS (N ≤ 172,082) at genome-wide significance P < 5 × 10–8 as instruments and clumped at linkage disequilibrium R2 < 0.3, leaving 13 and 6 moderately correlated instruments, respectively (Central Illustration, Supplemental Table 2).39
Cognition outcomes
We used 7 cognitive function outcomes (4 derived from UKB data): cognitive performance from the Social Science Genetic Association Consortium GWAS meta-analyzing UKB fluid intelligence verbal-numerical reasoning scores, and the Cognitive Genomics Consortium neuropsychological test data (N = 257,841) (standardized test score, mean = 0 ± 1)42; fluid intelligence verbal-numerical reasoning test score from the Medical Research Council Integrated Epidemiology Unit GWAS Pipeline using UKB data (N = 149,051) (test score, number of correct answers, mean = 6.16 ± 2.15)43; memory performance using UKB data (N = 112,065) (number of errors on matching tasks, mean = 4.06 ± 3.23)43; average reaction time in milliseconds for 4 matching trials using the UKB (N = 36,035) (mean = 555.08 ± 112.19 milliseconds)44; extremely high intelligence (1,238 cases selected from the top 0.03% of the intelligence distribution/8,172 controls)45; and 2 global measures of average cortical thickness (mean = 2.45 ± 0.11 mm) and total cortical surface area (mean = 169,647.43 ± 16,501.45 mm2) calculated from in vivo whole brain magnetic resonance imaging scans over 34 brain regions from a recent neuroimaging GWAS (N = 33,709) (Figure 1).46
Dementia outcomes
We used 4 dementia-related outcomes: AD progression score (a novel multimodal neuroimaging phenotype modeling disease progression as the time shift required to align biomarker trajectories from ADNI47); normalized hippocampal volume calculated as the ratio of bilateral hippocampal volume to total intracranial volume (mean = 0.0045 ± 0.00082) from ADNI; cerebral amyloid deposition, also from ADNI, calculated as the ratio of the standard uptake value in the cortical region and a composite reference region (white matter, whole cerebellum, brainstem, and pons) (mean = 0.78 ± 0.09)47; and LBD (2,591 cases: 4,027 controls) (Figure 1, Supplemental Table 1).48
Statistical analysis and interpretation of results
Because PCSK9 and HMGCR inhibitors are used to treat coronary artery disease (CAD), we used CARDIoGRAM- plusC4D CAD data (predominantly European ancestry, 60,801 cases: 123,504 controls) as positive controls to test PCSK9 and HMGCR instrument validity.49,50 First, we harmonized the drug target instrument exposures with all outcome datasets, and we performed correlated inverse variance weighted MR along with correlated MR Egger and correlated maximum likely- hood MR, generating heterogeneity and pleiotropy test statistics. Standardized correlated MR effect estimates per unit SD decrease in downstream biomarker LDL-C levels (SD = 38.7 mg/dL) proxying drug target gene inhibition are reported.
We caution against interpreting these study findings based solely on the P value threshold, which would penalize potentially important findings due to the inclusion of the 2 drug classes and 11 neurocognitive and dementia-related endpoints in this study (eg, the association of HMGCR inhibition on reduced cortical surface area) (Table 1).51,52 Therefore, we report the conventional P value threshold < 0.05 and emphasize evidence strength based upon the estimated size, the 95% CI, and the P value.
Values are mean ± SD or n unless otherwise indicated. Correlated Mendelian randomization (inverse variance weighted method) effect estimates in SD units per 1 SD decrease in SD low-density lipoprotein (LDL) (38.674 mg/dL) and OR per 1 SD decrease LDL for binary outcomes are reported, proxying effects of drugs inhibiting PCSK9 and statin target HMGCR.
HMGCR=3-hydroxy-3-methylglutaryl coenzyme A reductase; MRI = magnetic resonance imaging; NA = not available; NSNPs = number of single nucleotide polymorphisms used as instruments in each drug-target correlated Mendelian randomization analysis; PCSK9 = proprotein convertase subtilisin/kexin 9.
a. Standardized Mendelian randomization analysis regression coefficient.
b. Standardized test (fluid intelligence and neuropsychological tests).
c. Conventional statistical significance (P < 0.05).
d. Number of errors on pairs matching test (no time limit).
e. Mean time for 4 matching trials.
f. Number of correct answers on the 13-question test (timed).
g. Extremely high intelligence (top 0.03%).
h. Average global cortical thickness measures extracted from in vivo whole-brain T1-weighted brain MRI scans in 34 regions (defined by Desikan-Killiany atlas).
I. Global cortical surface area (total) measures extracted from in vivo whole-brain T1-weighted brain MRI scans in 34 regions.
Analyses were performed with R version 4.0.2 using the TwoSampleMR, and MendelianRandomization packages. 53,54
Results
Complete results are presented in Supplemental Table 3. To mimic pharmacological inhibition, all standardized correlated MR effect estimates were oriented to correspond to a unit SD reduction in LDL-C levels. First, as expected, in positive control analyses, our genetically proxied PCSK9 and HMGCR instruments reduced the risk for CAD (Table 1).
Regarding cognition, PCSK9 inhibition effect estimates were null for every cognitive-related outcome (Table 1). In contrast, genetic inhibition of HMGCR showed a harmful impact on cognitive performance score (beta = –0.082; 95% CI: –0.16 to –0.0079; P = 0.03), reaction time (beta = 0.00064; 95% CI: 0.00030-0.00098; P = 0.0002), and cortical surface area (beta = –0.18; 95% CI: –0.35 to –0.014; P = 0.03) (Table 1).
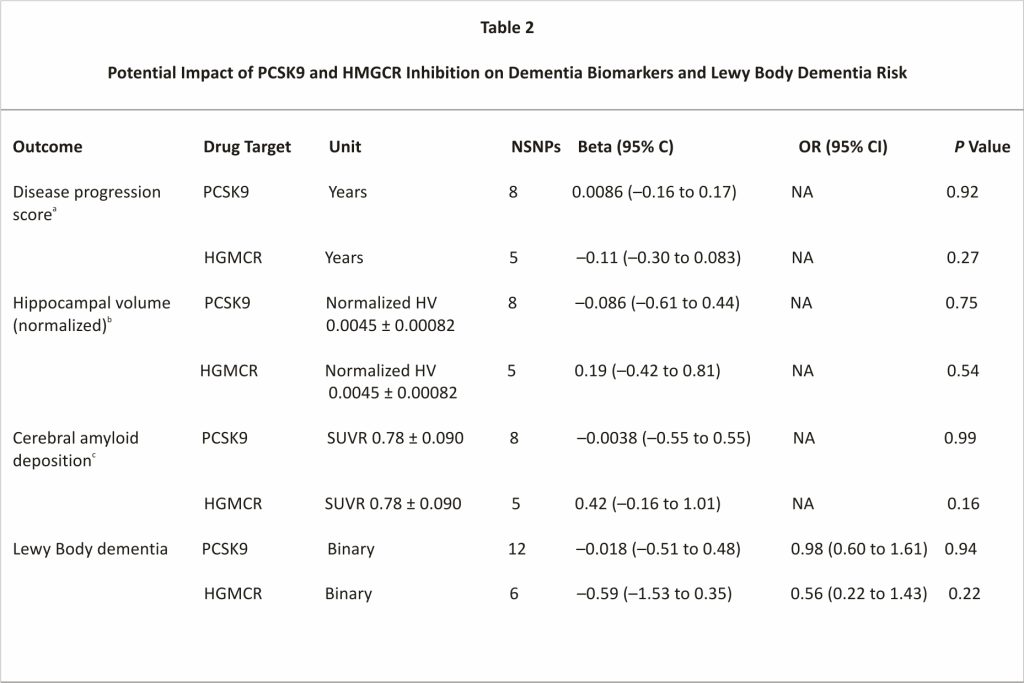
Further, we failed to find evidence that either PCSK9 or HMGCR inhibition impacted AD-related outcomes or LBD (Table 2). For most dementia-related variables, genetic inhibition estimates for PCSK9 were on the beneficial side of null with regression coefficients in the direction of reduced AD progression score, lower amyloid deposition levels, and lower LBD risk (Table 2). Overall, results were broadly consistent across MR methods; the Cochran Q test did not indicate heterogeneity, nor did the MR Egger intercept indicate directional pleiotropy, which improves the causal inference of the MR estimates (Supplemental Table 3).
Values are mean ± SD or n unless otherwise indicated. Correlated Mendelian randomization (inverse variance weighted method) effect estimates in SD units per 1 SD decrease in SD LDL (38.674 mg/dL) and OR per 1 SD decrease LDL for binary outcomes are reported, proxying effects of drugs inhibiting PCSK9 and statin target HMGCR.
CAD = cerebral amyloid deposition; HV = hippocampal volume; SUV = standard uptake value; SUVR = ratio of SUV in cortical region to that in the composite reference region; other abbreviations as in Table 1.
a. The shift in time required to align subject CAD and HV trajectories with long-term population evolution curves estimated from the disease progression modeling algorithm.
b. Bilateral hippocampal volume divided by total intracranial volume.
c. The ratio of SUVR is equal to the ratio of SUV in the cortical region to SUV in the composite reference region.
Discussion
Our drug-target MR analyses did not find evidence of adverse cognitive-related side effects related to inhibition of PCSK9 (Central Illustration), in concurrence with earlier safety assessments based on short-term clinical studies and genetic studies. 20,29,30,34 Crucially, where earlier studies—including large-scale clinical trials such as the EBBINGHAUS and FOURIER analyses—have examined the role of PCSK9 inhibitors in patients already taking statins, our analysis estimates the specific effect of PCSK9 inhibition on these outcomes, independent of concomitant drugs.20,27 We also report evidence of lasting genetic associations that mimic potentially long-lasting relationships between PCSK9 inhibition and adverse outcomes.
Although PCSK9i drugs have not generally been found to exhibit significant neuropsychiatric adverse effects in clinical trials, most of these trials either did not assess long-term outcomes (occurring at least 6 months post-treatment) or used PCSK9 inhibitors in combination with other pharmacological treatment (eg, statins). Further, PCSK9 inhibition is still a relatively new treatment approach, whereas statins have been used for the clinical management of hyper-cholesterolemia for decades, making any conclusive long-term safety evaluation challenging. In addition, researchers in several other disease areas are actively investigating PCSK9 inhibition as a novel therapeutic option, including stroke, AD, and alcohol-associated liver disease and alcohol use disorder, further highlighting the clinical need for long-term safety evaluations.55-59
With the novel approach of MR, we were able to simulate such a study by eliminating confounds and assessing patterns of genetic susceptibility to certain neurocognitive outcomes. The range of biological and clinical correlates of cognitive function and dementia used in this study broadens the scope and generalizability of these findings and provides key evidence for the neutral side effect profile of PCSK9 inhibitors. For example, our high-IQ variable can contextualize potential nonlinear lipid-lowering-cognition associations, and analyzing markers of dementia may elucidate possible age-specific associations that extend the findings reported in the existing literature.34 Although future clinical studies are required to evaluate whether localized neural PCSK9 expression impacts cognition or dementia, our findings support and extend the impact of existing short-term data showing that lowering LDL-C with PCSK9-based therapeutic agents has a neutral cognitive function profile.32 These findings are important to alleviate ongoing concern regarding the neurocognitive safety of PCSK9 inhibition that may be contributing to their under-use.6
In contrast, we identified potential relationships between sustained HMGCR inhibition and cognitive outcomes (reduced cortical surface area, worsened reaction time, and impaired cognitive performance) in line with some post-marketing surveillance, case reports, and patient survey-based analyses identifying cognitive impairment with statin use.60 Prior studies have also reported that cognitive side effects associated with statin use were reversed upon their discontinuation.60 Given that statins are among the most commonly prescribed classes of medication, our findings, taken together with these observations, suggest continued pharmacovigilance by clinicians and public health officials.
However, our finding of concurrent improved memory performance associated with the HMGCR instrument highlights the complexity of the HMGCR-cognition relationship.60 In parallel, we did not identify adverse or beneficial relationships of HMGCR inhibition with dementia-related outcomes, suggesting observationally reported reduction in dementia associated with statin use is due to confounding from factors such as differing patient characteristics, which may increase the likelihood of hypercholesterolemia while increasing risk of dementia and other neurocognitive diseases.61 Further, although we did not want to overlook potentially important clinical findings (and therefore used a nominal P value threshold of 0.05), several of the observed relationships between HMGCR inhibition and cognitive endpoints had P values that would not have surpassed a P value threshold adjusted for multiple testing. For example, the finding that HMGCR inhibition was associated with the reduced cortical surface area had a P value of 0.03 and would not be considered statistically significant after Bonferroni correction. Given the relatively small sample size of the cortical imaging data, these analyses should be replicated when larger samples become available. Relatedly, the finding that HMGCR inhibition was associated with slowed reaction time translated to an increase of 0.067 milliseconds per 1 SD decrease (38.7 mg/dL) in LDL-C. For comparison, previous work evaluating choice reaction time tasks among healthy adults aged 18-65 years found an increase in reaction time latency of 2.8 milliseconds per year, suggesting a small impact of HMGCR inhibition.62 Therefore, we emphasize that any potential adverse effects of HMGCR inhibition on neurocognition found in this study likely do not outweigh the cardiovascular benefits of statin use.
The ability to distinguish the precise side effect profiles of these therapeutics presents an opportunity to apply the principles of personalized medicine to metabolic diseases, including hypercholesterolemia. Personalized medicine refers to the practice of using individual characteristics of a patient, such as their demographics or genomic profile, to individually select medication likely to improve their specific outcome. Key to this capability is the understanding of how side effects and adverse outcomes differ in certain populations. If PCSK9 inhibitors and statins exert similar effects on LDL-C but have different side effect profiles, identifying these differences can help inform clinical prescription practices to maximize patient improvement while minimizing harmful side effects. To be clear, the overwhelming majority of the evidence suggests that both statins and PCSK9 inhibitors are safe and effective medications; our results do not indicate any worrisome tendency toward serious adverse effects of either class of drug. Nonetheless, some patients may respond better to statins or PCSK9 inhibitors, and physicians should consider these possibilities in their prescribing practices.
In practice, statins and PCSK9 inhibitors are imperfect substitutes for one another. Although both drugs have potent effects on cholesterol and are especially effective when combined, PCSK9 inhibitors are currently more than 100 times as expensive as statins, and cost-effectiveness poses a barrier to their widespread implementation as a treatment for hypercholesterolemia.63 The uncertainty regarding the side effect profiles of PCSK9 inhibitors is another potential cause for their underuse. Although side effects from PCSK9 inhibitors are generally regarded as mild, with the most common adverse outcomes being nasopharyngitis and injection site reactions, reports of potential cognitive side effects have cast a shadow over PCSK9 inhibitors’ potential as breakthrough treatments.64 Our findings may help alleviate this concern by providing genetic evidence that PCSK9 inhibitors are at least as safe regarding neurocognitive function as statins, the current standard of care. These results may help inform clinicians when prescribing drugs to treat hypercholesterolemia such as inclisiran, which have much longer effective durations than statins or PCSK9 monoclonal antibodies.
Our study capitalizes on the ability of MR to incorporate crucial biological and clinical variables related to cognitive function and dementia while maintaining high statistical power. Further, genomic analytic techniques such as MR can provide early evidence of long-term LDL-C modulation by drug targets; PCSK9 inhibitors have only recently been developed and long-term trial data are still lacking. Moreover, the consistency of our results across different MR methods accommodating varied sets of assumptions about genetic pleiotropy greatly strengthens the causal inference of our analysis.33
Study limitations
Our study is limited by several factors, primarily those owing to the assumptions and limitations of MR. First, MR is not a perfect substitute for authentic long-term randomized controlled trials of drugs such as inclisiran or future gene-editing therapeutics, which are still ultimately needed to rule out any adverse cognitive effects of sustained PCSK9 inhibition. MR can identify genetic associations with high statistical power, but it is subject to the limitations of the data from which it draws its conclusions. For instance, the UKB cohorts may not adequately represent the entire UK population or European individuals in general.65 There may also exist pleiotropic effects, which have been discussed in the context of the PCSK9 gene, and which could not be detected and subsequently corrected by the MR analyses used in this study.31 Moreover, as our analysis was limited to individuals of European ancestry, we caution against generalizing our findings to other populations. The ADNI outcome data on AD biomarkers is also small and may be underpowered enough to inadequately detect potential relationships. Therefore, these analyses using biometrics of AD should be repeated when larger datasets become available. Further, given the limited ability of PCSK9 inhibitors and statins to cross the blood-brain barrier and their mechanisms of action within the brain, our findings may not capture any tissue-specific relationships of PCSK9 expression that may be dysregulated in diseased brain states, which could have distinct roles in neurocognition that cannot be assessed using the drug-target MR framework.32,66 It is also important to consider the difference between pharmacological interventions’ biological and temporal context, such as statins and PCSK9 inhibitors, and the corresponding genetic defects assessed by genomic techniques such as MR. Although both statins and PCSK9 inhibitors are generally used by adults, genetic variants within the PCSK9 and HMGCR genes are determined at conception.67 Given the extensive role of cholesterol metabolism in myelination that occurs during early neural development, it may be that the marginally significant neurocognitive effects of HMGCR inhibition we observed are most relevant during the initial development of the nervous system.68 If so, statin use by adults may not share the same side effect profile or impact on the adult nervous system.
Conclusions
The apparent neutral impact of PCSK9 inhibition and possible adverse effects of HMGCR on the cognitive function we report should be interpreted with caution given the limitations of MR and the samples used and will need validation in long-term follow-up clinical studies. However, the data presented here are reassuring for clinicians considering PCSK9 inhibition as a treatment method for hypercholesterolemia who may be concerned about possible neurocognitive effects. The side effect profiles of PCSK9 inhibitors and HMGCR inhibitors are distinct from one another, and PCSK9 inhibition does not appear to share the observed risk for neurocognitive outcomes associated with HMGCR inhibitors. However, although the adverse effect estimates for HMGCR inhibition suggest that additional studies are warranted, these findings do not outweigh the cardiovascular benefits of statin use. Together, these findings should help alleviate ongoing fears of cognitive decline and other adverse neurocognitive effects that may be contributing to the underuse of these cardiovascular therapeutic classes.
Perspectives
COMPETENCY IN MEDICAL KNOWLEDGE:
Based on genome-wide association data, the statin drug target gene is associated with a slight adverse impact on cognitive function, reaction time, and cortical brain structure, whereas genetically proxied PCSK9 inhibition was not linked with neurocognitive side effects.
TRANSLATIONAL OUTLOOK
Long-term prospective studies that incorporate a range of neurocognitive measures are needed to understand differences in the impact of statins and PCSK9 inhibitors on cognitive function and the risk of dementia.
Funding Support and Author Disclosures
This work was supported by the National Institutes of Health (NIH) intramural funding [ZIA-AA000242 to F.W.L], and the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
AD: Alzheimer’s disease
GWAS: genome-wide association study
HMGCR: 3-hydroxy-3-methylglutaryl coenzyme A reductase
LBD: Lewy body dementia
LDL: low-density lipoprotein
MR: Mendelian randomization
PCSK9: proprotein convertase subtilisin /Kexin type 9
Acknowledgments
This research was facilitated by the Medical Research Council Integrative Epidemiology Unit (MRC-IEU, University of Bristol, United Kingdom), especially the developers of the MRC-IEU UK Biobank GWAS Pipeline. The authors thank the participants and investigators from the contributing studies; this would not be possible without them.
References
1. Roth G.A., Forouzanfar M.H., Moran A.E., et al. “Demographic and epidemiologic drivers of global cardiovascular mortality”. N Engl J Med 2015;372:1333-1341.
2. World Health Organization. “2021 global progress report on the implementation of the WHO Framework Convention on Tobacco Control”. Accessed July 6, 2022. https:// apps.who.int/iris/handle/10665/351735.
3. Holmes M.V., Richardson T.G., Ference B.A., Davies N.M., Davey Smith G. “Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development”. Nat Rev Cardiol 2021;18: 435-453.
4. Ray S., Jindal A.K., Sengupta S., Sinha S. “Statins: can we advocate them for primary prevention of heart disease?”. Med J Armed Forces India 2014;70: 270-273.
5. Reiner Ž. “Statins in the primary prevention of cardiovascular disease”. Nat Rev Cardiol 2013;10:453-464.
6. Ahmed H.M., Nissen S.E. “Nonstatin therapy for dyslipidemia”. Circ Res 2018;123: 1036-1038.
7. Musich S., Wang S.S., Schwebke K., Slindee L., Waters E., Yeh C.S. “Underutilization of statin therapy for secondary prevention of cardiovascular disease among older adults”. Popul Health Manag 2019;22:74-82.
8. Saeed A., Zhu J., Thoma F., et al. “Cardiovascular disease risk–based statin utilization and associated outcomes in a primary prevention cohort: insights from a large health care network”. Circ Cardiovasc Qual Outcomes 2021;14:e00 7485.
9. Samaras K., Makkar S.R., Crawford J.D., et al. “Effects of statins on memory, cognition, and brain volume in the elderly”. J Am Coll Cardiol 2019;74: 2554-2568.
10. Bradley C.K., Wang T.Y., Li S., et al. “Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry”. J Am Heart Assoc 2019;8:e011765.
11. Kelley B.J., Glasser S. “Cognitive effects of statin medications”. CNS Drugs 2014;28: 411-419.
12. Swiger K.J., Manalac R.J., Blumenthal R.S., Blaha M.J., Martin S.S. “Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects”. Mayo Clin Proc 2013;88:1213-1221.
13. Schultz B.G., Patten D.K., Berlau D.J. “The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms”. Transl Neurodegener 2018;7:5.
14. Chu C.-S., Tseng P.-T., Stubbs B., et al. “Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis”. Sci Rep 2018;8:5804.
15. Sabatine M.S. “PCSK9 inhibitors: clinical evidence and implementation”. Nat Rev Cardiol 2019;16:155-165.
16. Mullard A. “Nine paths to PCSK9 inhibition”. Nat Rev Drug Discov 2017; 16:299-301.
17. Shapiro M.D., Tavori H., Fazio S. “PCSK9”. Circ Res 2018;122:1420-1438.
18. Real F., Scott R., Somaratne R., et al. “Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial”. Circulation 2012;126:2408-2417.
19. Robinson J.G., Farnier M., Krempf M., et al. “Efficacy and safety of alirocumab in reducing lipids and cardiovascular events”. N Engl J Med 2015;372: 1489-1499.
20. Sabatine M.S., Giugliano R.P., Keech A.C., et al. “Evolocumab and clinical outcomes in patients with cardiovascular disease”. N Engl J Med 2017;376: 1713-1722.
21. Toth P.P., Descamps O., Genest J., et al. “Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies”. Circulation 2017;135:1819-1831.
22. U.S. Food and Drug Administration. “FDA approves add-on therapy to lower cholesterol among certain high-risk adults”. https://www.fda. gov/drugs/news-events-human-drugs/FDA-approves-add-therapy-lower-cholesterol-among-certain-high-risk-adults.
23. Leiter L.A., Teoh H., Kallend D., et al. “Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial”. Diabetes Care 2019;42:173-176.
24. Raal F.J., Kallend D., Ray K.K., et al. “Inclisiran for the treatment of heterozygous familial hypercholesterolemia”. N Engl J Med 2020;382:1520-1530.
25. Ray K.K., Wright R.S., Kallend D., et al. “Two phases 3 trials of inclisiran in patients with elevated LDL cholesterol”. N Engl J Med 2020;382:1507-1519.
26. Guedeney P., Giustino G., Sorrentino S., et al. “Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials”. Eur Heart J 2022;43:7: e17-e25. https://doi.org/10.1093/eurheartj/ehz 430.
27. Giugliano R.P., Mach F., Zavitz K., et al. “Design and rationale of the EBBINGHAUS trial: a phase 3, double-blind, placebo-controlled, multicenter study to assess the effect of evolocumab on cognitive function in patients with clinically evident cardiovascular disease and receiving statin background lipid-lowering therapy — a cognitive study of patients enrolled in the FOURIER trial”. Clin Cardiol 2017;40: 59-65.
28. Schwartz G.G., Steg P.G., Szarek M., et al. “Alirocumab and cardiovascular outcomes after acute coronary syndrome”. N Engl J Med 2018;379: 2097-2107.
29. Giugliano R.P., Mach F., Zavitz K., et al. “Cognitive function in a randomized trial of evolocumab”. N Engl J Med 2017;377: 633-643.
30. Mannarino M.R., Sahebkar A., Bianconi V., Serban M.C., Banach M., Pirro M. “PCSK9 and neurocognitive function: should it be still an issue after FOURIER and EBBINGHAUS results?”. J Clin Lipidol 2018;12:1123-1132.
31. Lohoff F.W. “Lipid-lowering drug effects beyond the cardiovascular system: relevance for neuropsychiatric disorders”. Int J Neuropsychopharm- acol 2018;21: 1076-1078.
32. O’Connell E.M., Lohoff F.W. “Proprotein convertase subtilisin/kexin type 9 (PCSK9) in the brain and relevance for neuropsychiatric disorders”. Front Neurosci 2020;14:609.
33. Davies N.M., Holmes M.V., Davey Smith G. “Reading Mendelian randomization studies: a guide, glossary, and checklist for clinicians”. BMJ 2018;362:k601.
34. Lyall D.M., Ward J., Banach M., et al. “PCSK9 genetic variants and cognitive abilities: a large-scale Mendelian randomization study”. Arch Med Sci 2021;17:241-244.
35. Williams D.M., Finan C., Schmidt A.F., Burgess S., Hingorani A.D. “Lipid lowering and Alzheimer disease risk: a Mendelian randomization study”. Ann Neurol 2020; 87:30-39.
36. Rosoff D.B., Davey Smith G., Mehta N., Clarke T.-K., Lohoff F.W. “Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study”. PLoS Med 2020;17:e1003410.
37. Petersen R.C., Aisen P.S., Beckett L.A., et al. “Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization”. Neurology 2010;74:201-209.
38. Sudlow C., Gallacher J., Allen N., et al. “UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age”. PLOS Med 2015;12: e1001779.
39. Willer C.J., Schmidt E.M., Sengupta S., et al. “Discovery and refinement of loci associated with lipid levels”. Nat Genet 2013;45:1274-1283.
40. Thompson P.M., Stein J.L., Medland S.E., et al. “The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data”. Brain Imaging Behav 2014;8:153-182.
41. Nikpay M., Goel A., Won H.-H., et al. “A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease”. Nat Gen 2015;47:1121-1130.
42. Lee J.J., Wedow R., Okbay A., et al. “Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals”. Nat Genet 2018;50:1112-1121.
43. Hemani G., Zheng J., Elsworth B., et al. “The MR-Base platform supports systematic causal inference across the human phenome”. eLife 2018;7:e34408.
44. Davies G., Lam M., Harris S.E., et al. “Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function”. Nat Commun 2018;9: 2098.
45. Zabaneh D., Krapohl E., Gaspar H.A., et al. “A genome-wide association study for extremely high intelligence”. Mol Psychiatry 2018;23:1226-1232.
46. Grasby K.L., Jahanshad N., Painter J.N., et al. “The genetic architecture of the human cerebral cortex”. Science 2020;367:eaay 6690.
47. Scelsi M.A., Khan R.R., Lorenzi M., et al. “Genetic study of multimodal imaging Alzheimer’s disease progression score implicates novel loci”. Brain 2018;141: 2167-2180.
48. Chia R., Sabir M.S., Bandres-Ciga S., et al. “Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture”. Nat Genet 2021; 53:294-303.
49. Gallego-Colon E., Daum A., Yosefy C. “Statins and PCSK9 inhibitors: a new lipid-lowering therapy”. Eur J Pharmacol 2020;878:173 114.
50. Nikpay M., Goel A., Won H.H., et al. “A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease”. Nat Genet 2015;47:1121-1130.
51. Sterne J.A., Davey Smith G. “Sifting the evidence what’s wrong with significance tests?”. BMJ 2001;322:226-231.
52. Amrhein V., Greenland S., McShane B. “Scientists rise against statistical significance”. Nature 2019;567:305-307.
53. Hemani G., Zheng J., Elsworth B., et al. “The MR-Base platform supports systematic causal inference across the human phenome”. Elife 2018;7: e34408.
54. Yavorska O.O., Burgess S. “Mendelian Randomization: an R package for performing Mendelian randomization analyses using summarized data”. Int J Epidemiol 2017;46:1734-1739.
55. Trial of PCSK9 Inhibition in Patients With Acute Stroke and Symptomatic Intracranial Atherosclerosis (TOPICAL-MRI). Accessed February 5, 2022. https://ClinicalTrials. gov/show/NCT05001984.
56. Abuelezz S.A., Hendawy N. “HMGB1/ RAGE/ TLR4 axis and glutamate as novel targets for PCSK9 inhibitor in high-fat cholesterol diet-induced cognitive impairment and amyloidosis”. Life Sci 2021;273:119310.
57. Lee J.S., O’Connell E.M., Pacher P., Lohoff F.W. “PCSK9 and the gut-liver-brain axis: a novel therapeutic target for immune regulation in alcohol use disorder”. J Clin Med 2021;10:1758.
58. Lee J.S., Mukhopadhyay P., Matyas C., et al. “PCSK9 inhibition as a novel therapeutic target for alcoholic liver disease”. Sci Rep 2019;9:17167.
59. Lohoff F.W., Sorcher J.L., Rosen A.D., et al. “Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder”. Mol Psychiatry 2018;23:1900-1910.
60. Schultz B.G., Patten D.K., Berlau D.J. “The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms”. Transl Neurodegener 2018;7:5.
61. drug report. “The 50 Most Commonly Prescribed Drugs in America And Their Average Price”. Accessed January 20, 2022. https://www.drugreport.com/50-commonly-prescribed-drugs-in-america/.
62. Woods D.L., Wyma J.M., Yund E.W., Herron T.J., Reed B. “Age-related slowing of response selection and production in a visual choice reaction time task”. Front Human Neurosci 2015;9:193.
63. Hlatky M.A., Kazi D.S. “PCSK9 Inhibitors”. J Am Coll Cardiol 2017;70:2677-2687.
64. Chaudhary R., Garg J., Shah N., Sumner A. “PCSK9 inhibitors: a new era of lipid-lowering therapy”. World J Cardiol 2017; 9:76-91.
65. Fry A., Littlejohns T.J., Sudlow C., et al. “Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population”. Am J Epidemiol 2017;186:1026-1034.
66. Schmidt A.F., Finan C., Gordillo-Marañón M., et al. “Genetic drug target validation using Mendelian randomisation”. Nat Commun 2020;11:3255.
67. Sanderson E., Glymour M.M., Holmes M.V., et al. “Mendelian randomization”. Nat Rev Methods Primers 2022;2:6.
68. Saher G., Brügger B., Lappe-Siefke C., et al. “High cholesterol level is essential for myelin membrane growth”. Nat Neurosci 2005;8:468-475.
Credits: Rosoff D.B., Bell A.S., Jung J., Wagner J., Mavromatis L.A., Falk W. Lohoff. “Mendelian Randomization Study of PCSK9 and HMG-CoA Reductase Inhibition and Cognitive Function”. J Am Coll Cardiol. 2022 80(7): 653-662, Online publication date: 16-Aug-2022.