Natalie S. Joe, Inês Godet, Nubaira Milki, Noor U. I. Ain, Harsh H. Oza, Gregory J. Riggins & Daniele M. Gilkes
Abstract
Breast cancer is the most diagnosed cancer among women. Approximately 15–20% of all breast cancers are highly invasive triple-negative breast cancer (TNBC) and lack estrogen, progesterone, and ERBB2 receptors. TNBC is challenging to treat due to its aggressive nature with far fewer targeted therapies than other breast cancer subtypes. Current treatments for patients with TNBC consist of cytotoxic chemotherapies, surgery, radiation, and in some instances PARP inhibitors and immunotherapy. To advance current therapeutics, we repurposed mebendazole (MBZ), an orally available FDA-approved anthelmintic that has shown preclinical efficacy for cancers. MBZ has low toxicity in humans and efficacy in multiple cancer models including breast cancer, glioblastoma multiforme, medulloblastoma, colon cancer, and pancreatic and thyroid cancer. MBZ was well-tolerated in the phase I clinical trials of adults recently diagnosed with glioma. We determined that the half-maximal inhibitory concentration (IC50) of MBZ in four breast cancer cell lines is well within the range reported for other types of cancer. MBZ reduced TNBC cell proliferation, induced apoptosis, and caused G2/M cell cycle arrest. MBZ reduced the size of primary tumors and prevented lung and liver metastases. In addition, we uncovered a novel mechanism of action for MBZ. We found that MBZ reduces integrin β4 (ITGβ4) expression and cancer stem cell properties. ITGβ4 has previously been implicated in promoting “cancer stemness,” which may contribute to the efficacy of MBZ. Collectively, our results contribute to a growing body of evidence suggesting that MBZ should be considered as a therapeutic to slow tumor progression and prevent metastasis.
Introduction
Breast cancer (BC) is the most common neoplasm among women and comprises more than 500,000 deaths worldwide, with ~ 2.0 million new cases diagnosed each year 1. Breast cancer is characterized by molecular subtypes 2,3,4,5 and histological subtypes 6,7. Pathologists use immuno- histochemical (IHC) staining to determine the presence or absence of two hormone receptors (HR), the progesterone receptor (PR) and the estrogen receptor (ER), as well as the human epidermal growth factor receptor 2 (HER2) 6,7. While there are targeted therapies for patients with HR+/HER2−, HR+/HER2+ and HR−/HER2+ disease, triple-negative (TN) BC lacks HR and HER2, rendering ER and HER2 therapies ineffective 8. TNBC is aggressive and often leads to bone, brain, liver, and lung metastases 9,10, emphasizing that new therapeutics are critically needed to improve patient outcomes.
Mebendazole (MBZ) was approved by the US Food and Drug Administration (FDA) in 1971 as an anthelmintic drug to treat parasitic infections 11 but has recently been shown to have preclinical efficacy 12 in the treatment of pancreatic 13, lung 14,15, thyroid 16, breast 17, meningioma 18, brain 19,20,21, melanoma 22, and colorectal 23 cancers. The first mechanism of action reported for MBZ was tubulin disruption. Tubulin is a common target of chemotherapy drugs such as paclitaxel, colchicine, and vincristine, which explains MBZ’s anti-proliferative effect in cancer cells 24. Other reported tumor suppressive mechanisms of MBZ include inhibiting angiogenesis and inducing apoptosis through B cell lymphoma 2 (BCL-2) and caspase-3-dependent mechanisms 21,25,26. MBZ treatment also induces G2/M cell cycle arrest and apoptosis leading to reduced lung metastasis in papillary and anaplastic thyroid cancer preclinical models 16. In addition, a phase I clinical trial of adults with newly diagnosed high-grade glioma demonstrated that MBZ is safe and tolerable 27. The promising results prompted us to determine whether MBZ could be used for the prevention or treatment of breast cancer metastasis.
Given the lack of treatment options and the propensity for TNBC to metastasize, we hypothesized that MBZ might be safe and effective for the prevention and treatment of metastasis. As reported for other cancer types, we find that MBZ promoted apoptosis and G2/M cell cycle arrest 13,16 but reduced the expression of genes involved in angiogenesis and cell migration 12,16,19,23,26,28,29. For the first time, we demonstrate that MBZ reduced lung metastasis and eliminated liver metastases in mouse models of TNBC. TNBC tissue has been reported to be enriched for cancer stem cells (CSCs) which contributes to the aggressive nature of the disease. Cancer cell stemness is known to be regulated by ITGβ4, and cancer stem cells (CSCs) have been shown to contribute to metastasis 30. We show that treatment with MBZ led to a dramatic reduction in integrin β4 (ITGβ4) expression both in vitro and in cancer cells harvested from tumor-bearing mice following MBZ treatment. Taken together, our results show that mebendazole prevents distant organ metastases in TNBC models, in part by decreasing ITGβ4 expression and cancer stemness.
Methods
Cell lines and cell culture
Mycoplasma-free breast cancer cell lines, MDA-MB-231 (ATCC® HTB-26™) and 4T1 (ATCC® CRL-2539™) cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM (MDA-MB-231; Sigma-Aldrich) or RPMI-1640 (4T1; Sigma-Aldrich) with 10% fetal bovine serum (FBS; Corning) and 1% penicillin /streptomycin (P/S) (Invitrogen). SUM159 and 4T1-luciferase-tagged (4T1-Luc) cells were kindly provided by the Sukumar lab and were cultured in Ham’s F12 medium supplemented with 5% FBS, 1% P/S, and 5% insulin/ hydrocortisone (SUM159) or in DMEM with 10% FBS and 1% P/S (4T1-Luc). Cells were maintained in a humidified environment at 37 °C and 5% CO2. MMTV-PyMT cells were derived from a tumor excised from a female triple-transgenic mouse as previously described 31 and maintained in 50% DMEM, 50% DMEM/F12, 10% FBS, 5% insulin, and 1% P/S. Fluorescent MDA-MB-231 cells were developed and maintained as previously described. 32
Proliferation assays
Half-maximal inhibitory concentration (IC50) values were obtained for MMTV-PyMT, MDA-MB-231, 4T1, and SUM159 cells by seeding 1000 cells/well in a 96-well plate for 24 h (h) and then treated with 0.01–500 µM of mebendazole (MBZ) or less than 1% dimethyl sulfoxide (DMSO) as vehicle control. After 48 h of treatment, PrestoBlue (Thermo Fisher) was added to achieve a 10% (v/v) concentration in each well, incubated for 4 h, and fluorescence was measured using a Cytation5 (BioTek Instruments). The IC50 was calculated using a nonlinear fit log vs. response model. To calculate % cell survival, cells were fixed with 70% ethanol, stained with DAPI, and imaged using a Cytation 5 (BioTek Instruments) equipped with an Olympus–UPLFLN 4XPh phase objective and DAPI filter. A 4 × 3 montage was used to capture the entire area of each well, and NIS Elements software (Nikon Instruments Inc.) was used to threshold the DAPI positive area of the image which is presented as % survival.
Colony formation assays
MDA-MB-231, 4T1, and SUM159 cells were plated in a 24-well plate (250 cells/well) and exposed to 0.01–1 µM of MBZ or < 1% DMSO as vehicle control for 10–14 days (refreshed every 3 days) or pre-treated with MBZ for 48 h and then seeded in plates without further drug treatment. The cells were washed with 1 × phosphate-buffered-saline (PBS), fixed with 4% paraformaldehyde (PFA) in PBS for 15 min (min), and washed again with PBS. Crystal violet solution (1% (w/v) crystal violet diluted in water containing 20% methanol) was added to each well and incubated for 10 min at room temperature. Wells were washed with distilled water and dried. Well, plates were imaged using a Cytation 5 (BioTek Instruments) equipped with an Olympus–UPLFLN 4XPh phase objective in a color bright field. A 4 × 3 montage of the acquired images was used to capture an image of the entire well. Colonies were quantified by manually counting individual colonies in each well.
Cell cycle analysis
MDA-MB-231 cells were treated with 0.25 µM, 0.5 µM, 1 µM, and 5 µM of MBZ or DMSO (vehicle control) for 48 h, washed with PBS, fixed in 70% ethanol, and pelleted and stained with 100 µg/mL RNase and 50 µg/mL of propidium iodide in the dark at 4 °C overnight. The distribution of cells in the G1, S, and G2/M phases was determined by flow cytometry using a CytoFLEX flow cytometer (Beckman Coulter), and data analysis was performed using FlowJo software V10.
Western blotting
Cell homogenates were prepared from MDA-MB-231 and SUM159 cell lines or primary tumors formed by MDA-MB-231 cells lysed in IGEPAL CA-630 buffer (150 mM sodium chloride (NaCl), 1% IGEPAL CA-630, 50 mM Tris–HCL, pH 8.0, protease and phosphatase inhibitors) for 10 m on ice. The lysate was centrifuged for 10 min at 13,000 g at 4 °C, and the supernatant was collected. Proteins were fractionated by a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane for 30 min using a Trans-blot Turbo (Bio-Rad). The membranes were blocked in 5% milk (% w/v) in 1 × Tris-buffered saline and 0.1% Tween-20 (TBS-T) and incubated overnight in primary antibodies at a dilution of 1:1000 at 4 °C. The membranes were washed three times in TBS-T and incubated in HRP-conjugated secondary antibodies (Cell Signaling Technology) followed by three additional washes in TBS-T. The chemiluminescence signal was detected using an AZURE C300 (Azure™ Biosystems) after incubating the membrane with ECL (Perkin Elmer). A list of antibodies can be found in Additional file 1: Table S1.
Transwell migration assays
Transwell invasion assays were performed using Costar Transwell cell culture inserts (Corning) with an 8-μm pore size PET membrane in a 24-well plate. The lower chambers of the transwell plates were filled with 500 μL of cell-appropriate culture media. MDA-MB-231 or SUM159 cells were pre-treated with 0.5 or 1 μM of MBZ for 48 h. The cells (20,000/well) were resuspended in 150 μL of medium containing only 1% FBS and placed into the upper well. After 24 h, cells in the upper chamber were removed with a cotton swab, and those that migrated through the pores on the lower surface were fixed in 100% ethanol (EtOH) and stained in 0.2% crystal violet dye. Five random fields of each pore were imaged using a Cytation 5 (BioTek Instruments) equipped with an Olympus–UPLFLN 10XPh phase objective. For MDA-MB-231 cells, the percent area and total area were quantified using ImageJ software using a custom macro with the following steps: Each image was converted to 16-bit, the scale was converted from pixel to μm, the background was subtracted using a rolling ball radius of 10 pixels and light background, and a threshold was set to highlight crystal violet positive areas of the wells. The macro was altered to include the color threshold feature, the “hue” slider was used to highlight the cell area, and images were processed at 8-bit to quantify the % area for SUM159 cells.
Wound-healing assays
MDA-MB-231 and SUM159 cells were cultured in a 12-well plate (100,000 cells/well) and allowed to reach near confluence within 48 h. Linear scratches of equal width were introduced using a 200 µL pipette tip. Fresh media containing 0.25 µM, 0.35 µM, 0.5 µM MBZ, or DMSO (vehicle control) was added. Five images of the wounded area were taken at 12 and 24 h using a Cytation 5 (BioTek Instruments) equipped with an Olympus–UPLFLN 4XPh phase objective. A montage of the entire well was constructed by stitching individual images. The average healing speed and area covered were quantified using ImageJ software. The following macro was created: Images were converted to an 8-bit format, the scale was set with the appropriate pixel to μm conversion, a Gaussian blur of radium 5 pixels was applied, and a threshold was applied using the measure tool to highlight only the gap created by the wound. The macro code calculates the wound area covered in μm2; this value was then divided by the total hours of the experiment (12 h or 24 h).
DNA extraction from mouse tissues
Mouse liver or lung tissues were placed in Genomic DNA lysis buffer (1 M Tris, pH 8.0, 5 M NaCl, 0.5 M EDTA, 10% Tween-20, 10% NP-40, and 40 μg of proteinase K) at 55 °C for 3 h with intermittent vortex. Proteinase K was inactivated with a 2 min incubation at 95 °C. One volume of phenol/ chloroform/isoamyl alcohol (25:24:1) (Sigma) was added to each sample, followed by a 20 s vortex. Samples were centrifuged for 10 min at 13,000 × g, and the upper aqueous phase was collected. Glycogen (20 μg), 0.1 volume of 2.5 M NaCl, and 2 × volume of 100% EtOH were added, and samples were incubated for 20 min at − 20 °C. The samples were spun at 13,000 × g for 15 min to pellet the DNA followed by one wash with 70% EtOH. The sample was spun at 13,000 × g for 5 min, the remaining liquid was decanted, and the DNA pellets were air-dried. DNA pellets were resuspended in Dnase/ RNase-free water. To assess the amount of human DNA content in the mouse lung and liver, the cycle threshold value of human hexokinase 2 (HK2) measured by RT-PCR was normalized to the DNA concentration of the sample (ranging from 180 to 220 ng/µL). The result for each sample was then normalized to the average value of the control group.
Reverse transcription and quantitative polymerase chain reaction (qPCR)
Total RNA was extracted using TRIzol (Invitrogen) followed by cDNA synthesis using Promega’s GoScript™ Reverse Transcriptase. qPCR analysis was conducted on the CFX96 Real-Time PCR detection system (Bio-Rad) using SYBR Green qPCR master mix (Bio-Rad). The expression of each target mRNA relative to 18S rRNA control was calculated based on the cycle threshold (Ct) as 2 − Δ(ΔCt), in which ΔCt = Ct (target mRNA) − Ct (18S rRNA), and Δ(ΔCt) = ΔCt (treatment) − ΔCt (control). Primer sequences are listed in Additional file 1: Table S2. (Figs. 1, 2, 3, 4, and 5).
RNA sample preparation and sequencing
MDA-MB-231 and SUM159 cells were collected from the culture, and subsequently, total RNA was extracted. Following RNA purification, samples were confirmed to have a RIN value > 9.0 using an Agilent Bioanalyzer. Total RNA was further qualified upon receipt of Novogene (Sacramento, CA). The samples were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). RNA purity was checked using the NanoPhotometer spectrophotometer (IMPLEN, CA, USA). One μg of RNA was used as input for mRNA library preparation. mRNA was purified from total RNA using poly-T oligo-attached magnetic beads followed by double-stranded cDNA synthesis. The cDNA fragments were adenylated, and sequencing adaptors were added. Each 150–200 bp length fragment was purified, and PCR was performed per Novogene-established protocols. Sequencing was performed on a NovaSeq 6000 system with a 150-bp paired-end run by Novogene (Sacramento, CA). The reads were mapped to Homo Sapiens (GRCh38/hg38) using STAR (v2.5) with the parameter mismatch set to 2. Quantification was done using HTSeq (v0.6.1) software with the parameter-m union. Differentially expressed genes were identified using EdgeR (v3.16.5) with padj < 0.005 and [log2(FoldChange)] > 1. FASTQ files and read counts have been uploaded to GEO with the accession number: GSE190845. For the gene ontology enrichment analysis, the p-value cutoff was set at p < 0.05. In order for a GO pathway to be included in Additional file 2: Fig. S5, the pathway had to be enriched (p < 0.05) in both the SUM159 and MDA-MB-231 cell lines. Differentially expressed genes (DEGs) consisted of genes that increased or decreased with a fold change of 1.5 or higher. Genes meeting these criteria were included in the VENN diagram in Fig. 6. Additional file 3 (SuppDataRNAseq.xls) includes all GO enrichment scores and differential expression values for each cell line.
Immunofluorescence staining
Immunofluorescence staining to detect ITGβ4 was performed on 10-μm-thick tissue cryo-sections after rehydrating them by immersing them into 1 × phosphate-buffered saline and 0.1% Tween-20 (PBS-T). All slides were then blocked with 2% BSA for 60 min and incubated overnight at 4 °C with a primary antibody against ITGβ4 (50–1049-80, Invitrogen) (dilution at 1:200). The next day, slides were washed 3× with PBS-T and then incubated with DAPI (1 ug/mL) for 15 m at room temperature. Sudan Black (0.1% w/v) was applied to the slides for 25 min to quench autofluorescence followed by washing in PBS-T. ITGβ4 and DAPI were visualized in Cy5 and DAPI channels, respectively, using Cytation 5 (BioTek Instruments) equipped with an Olympus–UPLFLN 10XPh phase objective.
Mammosphere formation assay
Fluorescently tagged MDA-MB-231 cells in culture or freshly resected from tumors that were processed (enzymatic digestion in 2 mg/mL collagenase for 1 h at 37 °C with orbital shaking at 160 rpm) were passed through a cell strainer (70 μm) and plated at 2,000 cells/well in 12-well plates previously coated with polyHema (12 g/L in 95% EtOH) and air-dried for 48 h, with 2 mL of mammosphere formation media per well (MammoCult basal medium (human) containing 4 μg/mL heparin, and 0.48 μg/mL hydrocortisone) (STEMCELL Technologies; Vancouver, BC, Canada). Fluorescent images were captured using a Cytation 5 (BioTek Instruments) equipped with an Olympus –UPLFLN 4XPh phase objective. A montage of the entire well was constructed by stitching individual images. The total number and average size of each mammosphere were then calculated using Gen5 software (BioTek instruments) automated counting algorithm.
Fluorescence-activated cell sorting (FACS)
MDA-MB-231 and SUM159 cells were treated with fresh media containing 0.05 µM, 0.125 µM, 0.5 µM, 1 µM, or 5 µM MBZ or DMSO (vehicle control) for 72 h. Cells were then trypsinized, resuspended in culture media, washed with 1xPBS, and collected in FACS buffer (1xPBS, 1%BSA, 0.5 mM EDTA, and 25 μg/ml DNase). Live cells were stained with APC-conjugated antibody against ITGβ4 (50-1049-80, Invitrogen) diluted at 1:300 for 30 min on ice. Sytox™ Blue (Invitrogen, dilution 1:300) was added immediately before analyzing each sample using flow cytometry. APC (ITGβ4) was detected in the FL-4 channel, and FITC (Sytox™ Blue) was detected in the FL-1 channel. Or cells were stained with conjugated antibodies against ITGβ4, CD44, and CD24 diluted at 1:300 for 30 min on ice. APC (ITGβ4) was detected in the FL-4, FITC (CD44) was detected in the FL-1 channel, and PE (CD24) was detected in the FL-2 channel. Single-stained cells were used for compensation controls. Data were analyzed using FlowJo V10 software (Tree Star, Inc.). For primary tumors that were sorted, tumors were subjected to enzymatic digestion prior to staining and ultimately resuspended in sorting buffer (1xPBS, 1%BSA, 0.5 mM EDTA, and 25 μg/ml DNase). Samples were sorted on an SH800 cytometer (Sony) into ITGβ4+ or ITGβ4− expressing populations directly into media. APC (ITGβ4) was detected in the APC channel, and Alexa Fluor 405 (Sytox™ Blue) was detected in the Pacific Blue channel. Single-stained cells were utilized as compensation controls. A list of antibodies can be found in Additional file 1: Table S1.
Breast cancer patient-derived xenograft (PDX) maintenance
The HCI-001 PDX was kindly provided by the Zahnow Lab and developed by the Welm lab (https://uofuhealth. utah.edu/huntsman/labs/welm-labs/ research.php). The individual demographical data can be found in the Baylor College of Medicine PDX Portal (https://pdxportal.research. bcm.edu/). The tumor fragments from the HCI-001 PDX were maintained in NOD-SCID Gamma (NSG) mice. When tumors reached the maximum diameter approved by Johns Hopkins University Animal Care and Use Committee (ACUC), they were collected and re-implanted into new mice as ~ 1 mm size fragments or cryopreserved. The initial tumor was termed “p.x+1,” and passages were tracked. For experiments, primary tumors were manually dissociated and incubated for 1 h at 37 °C in 2 mg/mL collagenase on an orbital shaker and then digested with DNase (0.4 U/ml) (Sigma-Aldrich) for 5 min at room temperature. Next, the tumor-dissociated cell suspension containing organoids was centrifuged and resuspended in fresh media followed by differential centrifugation (×4) at 520 g for 2 s. Finally, the cell suspension was strained through a 100 μm Nylon filter, counted, and then, 5000 organoids were re-implanted into each recipient NSG mouse. This process has been described in-depth previously 33.
In vivo orthotopic breast cancer models
All animal research complied with relevant ethical regulations within protocols approved by the Johns Hopkins University ACUC. Female 5- to 7-week-old NOD-SCID Gamma (NSG) mice or BALB/c mice were anesthetized by the intraperitoneal injection (i.p.) of 100 mg/kg ketamine and 16 mg/kg xylazine. MDA-MB-231 cells (2 × 106; NSG), 4T1-luciferase-tagged cells (500; BALB/c), or HCI-001 tumor organoids (5000; NSG) were injected into the mammary fat pad closest to the second nipple. Tumor removal surgery was performed 5.5 weeks (MDA-MB-231 model only) post-tumor implantation as described previously 34. At the end of the experiment, tumors, livers, and lungs were excised. A portion of each organ was first formalin-fixed (Sigma-Aldrich) overnight and then saturated in 30% sucrose (Sigma-Aldrich) at 4 °C overnight. Each organ was then placed into a cryomold and covered completely with OCT media (Fisher Scientific). After flash freezing the OCT-embedded organ in liquid nitrogen, they were sectioned onto Superfrost Plus Microscope Slides (Fisher Scientific) using a cryotome CM11000 (Leica). A second portion of the same tumor, liver, and lung was flash-frozen and processed for DNA or RNA extraction. Primer sequences are listed in Additional file 1: Table S2. For mice injected with the HCI-001 PDX, rather than embedding in OCT, the lungs from NSG mice were inflated with agarose, formalin-fixed, embedded in paraffin, and H&E stained.
Bioluminescence assay to measure the metastatic burden
Intratumoral bioluminescence was measured weekly throughout the study by injecting the BALB/c mice bearing 4T1-Luciferase expressing tumors with 2 µg/mouse (150 µL) of D-luciferin potassium salt (Gold Bio) diluted in 1 × PBS. Mice were then briefly anesthetized with Fluorido (Isoflurane, USP) and imaged using a Xenogen imaging system (IVIS 200, equipped with Living Image Software) within 10 min of luciferin injection, as described previously 20. All tumor bioluminescence values were expressed as radiance (photons.s-1.cm2.103). At the end of the study, lungs and livers were excised and immediately imaged to detect bioluminescence.
Limiting dilution assays (LDA)
Animal experiments complied with Johns Hopkins ACUC protocols. In vitro pre-treatment (Fig. 7E): MDA-MB-231 cells were grown in fresh media containing 1 µM MBZ or DMSO (vehicle control) for 72 h. Cells were trypsinized, counted, and resuspended at 106, 105, 104, or 103 cells per 100 µL in a 1:1 ratio of Matrigel to PBS. Nude mice (female, 6–8 weeks of age) were implanted in the 2nd, 3rd, 4th, and 5th mammary fat pads on either side of the mice. The right mammary fat pads were injected with cells treated with vehicle control, and the left mammary fat pads were injected with MBZ-treated cells as follows: 2nd—106 cells, 3rd—105 cells, 4th—104, and 5th—103. In vivo pre-treatment (Fig. 7G): MDA-MB-231 cells were injected into the right and left mammary fat pads of three NSG mice. Five days post-injection, mice were treated with 30 mg/kg MBZ in a sesame oil suspension (N = 2) or sesame oil alone (N = 1) 4 × per week. After 30 days, tumors and lungs were excised for further processing. Primary tumors underwent enzymatic digestion in 2 mg/mL collagenase for 1 h at 37 °C at 160 g and strained through a 70 µm filter. Cells were counted and resuspended at 0.8 × 106, 0.8 × 105, 0.8 × 104, or 0.8 × 103 cells per 100 µL in a 1:1 ratio of Matrigel to PBS. Secondary recipient nude mice (female, 6–8 weeks of age) were implanted in the 2nd, 3rd, 4th, and 5th mammary fat pad on either side of the mice. The right mammary fat pads were injected with tumor-derived cells from NSG mice treated with sesame oil alone. In contrast, the left mammary pads were injected with the following dilutions of tumor-derived cells from NSG mice treated with 30 mg/kg MBZ in a sesame oil suspension as follows: 2nd—0.8 × 106 cells, 3rd—0.8 × 105 cells, 4th—0.8 × 104, and 5th—0.8 × 103. In both the in vitro and in vivo pre-treated LDA experiments, mouse weights, and tumor growth were regularly measured. At the end of the experiment, tumors were excised and weighed. Stem cell frequency was calculated based on tumor incidence in all treatment groups using ELDA: Extreme Limiting Dilution Analysis. 35
Mebendazole in vivo dosages
MBZ pure polymorph C powder (Batch No. 1180916-WC, K A Malle Pharmaceuticals LTD) was utilized in all in vitro experiments and utilized for oral gavage dosing of mice. The mice were weighed on a weekly basis with their body masses averaged in treatment groups to calculate the 30 mg/kg MBZ dosage in a sesame oil (Sigma-Aldrich) suspension. The mice were treated 4× a week for the experiment and exposed to a weekly dosage of 120 mg/kg of MBZ. A second route of MBZ treatment utilized was MBZ incorporated into mouse feed. Research Diets, Inc. (New Brunswick, NJ) formulated rodent diets with 45% Kcal fat and modified version with MBZ at 215 ppm (0.18% MBZ of the total mixture) and 250 ppm (0.22% MBZ of the total mixture). The ingredients are as follows: protein, carbohydrate, fat, casein, l-cystine, corn starch, maltodextrin 10, sucrose, cellulose, soybean oil, lard, mineral mix S10026, dicalcium phosphate, calcium carbonate, potassium citrate, vitamin mix V10001, choline bitartrate, FD&C yellow dye #5, and red dye #40. The mice were weighed, and the food consumed was monitored to calculate the dosages administered of MBZ. The mice were able to eat the feed incorporated with MBZ seven days a week and on average consumed the following dosages: 215 ppm–152 mg/kg MBZ weekly intake (25 mg/kg/ day) and 250 ppm–197 mg/kg MBZ weekly intake (32 mg/kg/day). These routes of MBZ dosages differ in dose rates since the mice exposed to the feed have consistent exposure, unlike the oral gavage-dosed mice that are given treatments 4 × per week.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM), and statistical analysis was performed using GraphPad Prism 9 with statistical tests appropriate for each experimental setup. Each comparison of two variables was performed using an unmatched two-way ANOVA with Bonferroni multi-comparison tests. A comparison of a single variable measured in a sample at two different locations was performed via paired two-tailed Student’s t-test. A comparison of “yes” or “no” metastases categorical data was performed via a chi-squared test. Significance levels are reported as ****P < 0.0001, ***P < 0.001, **P < 0.01 *P < 0.5.
Results
Treatment of triple-negative breast cancer (TNBC) cells with mebendazole (MBZ) inhibited proliferation and colony formation
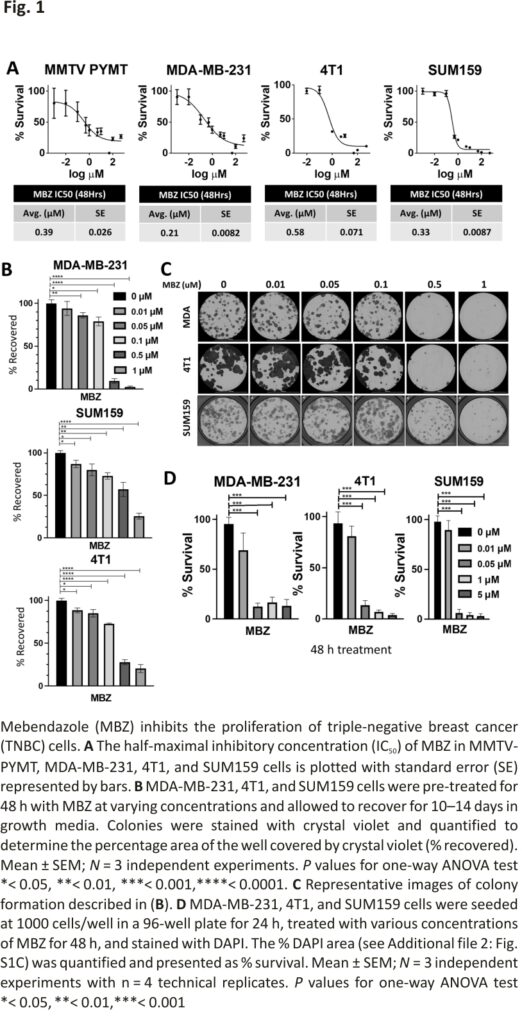
The ability of MBZ to decrease cell survival has been tested in thyroid, glioblastoma, breast, and colon cancer cell lines 15,17,23 with IC50 values ranging from 0.1 μM – 0.8 μM. We determined the IC50 values for commonly cultured BC cell lines. In mouse BC cells, MMTV-PyMT, and 4T1, the IC50 values were 0.39 μM and 0.58 μM, respectively. For human TNBC cell lines, MDA-MB-231, and SUM159, the MBZ IC50 values were 0.21 μM and 0.33 μM, respectively (Fig. 1A). This is consistent with the IC50 value for SUM159 cells previously reported 17 and demonstrates that the IC50 for BC cells is consistent with other cancer types (0.1–0.8 μM) (Additional file 2: Fig. S1A).
Next, we assessed the ability of BC cell lines treated with MBZ to form colonies using two different approaches. First, TNBC cells were pre-treated with a range of MBZ concentrations (0.01 μM, 0.05 μM, 0.1 μM, 0.5 μM, or 1 μM) or with DMSO as the vehicle control for 48 h. The cells were then seeded at 250 cells/well, and colonies were allowed to recover and form for 10–14 days (Fig. 1B, C). Then, in a second approach, BC cell lines were seeded at 250 cells/well and continually treated with a range of MBZ concentrations (0.01 μM, 0.05 μM, 0.1 μM, 0.5 μM, or 1 μM) or with a DMSO vehicle control for 10–14 days (Additional file 2: Fig. S1B-E). Whereas the first approach measured the ability of cancer cells that have survived treatment to recover and form colonies, the second approach measured the ability of cells to survive during treatment. Both methods showed that MBZ inhibited the ability of TNBC cells to form colonies. In addition, treating cells with MBZ for 48 h after initial seeding decreased cell proliferation in short-term assays (Fig. 1D; Additional file 2: Fig. S1F). Taken altogether, our results showed MBZ treatment inhibits short-term and long-term proliferation of BC cell lines and survival after treatment.
MBZ causes the induction of G2/M cell cycle arrest in human TNBC cells and apoptosis
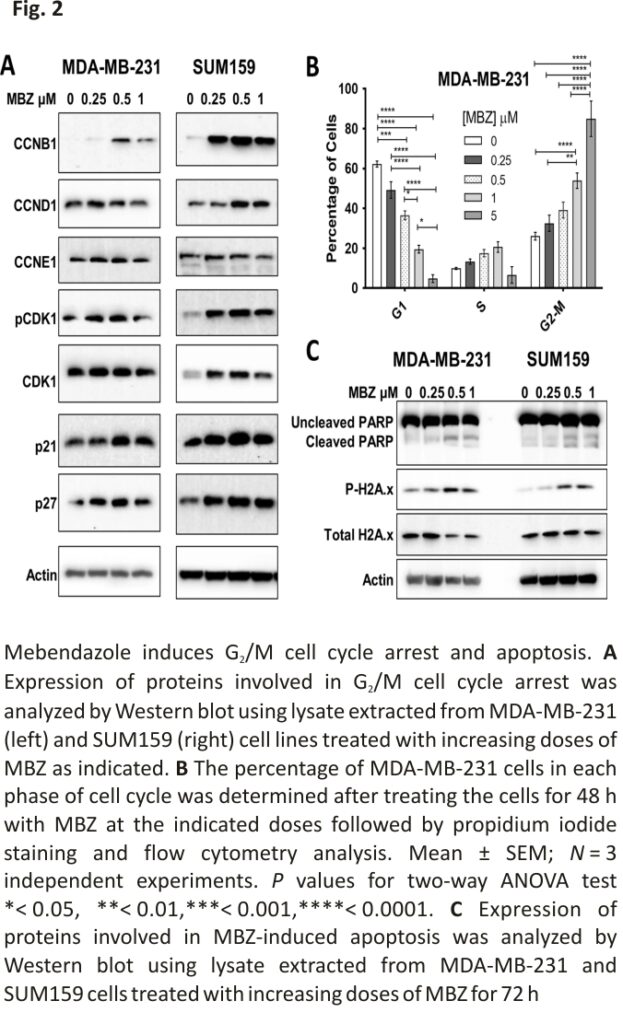
MBZ has previously been reported to induce G2/M cell cycle arrest and apoptosis in the thyroid, glioblastoma, breast, and colon cancer cell lines 16,17,26,27,29. Therefore, we investigated the effect of MBZ treatment on proteins involved in cell cycle arrest and apoptosis. In MDA-MB-231 and SUM159 cell lines, MBZ induced the cyclin-dependent kinase inhibitors p21 and p27. In addition, MBZ treatment increased G2/mitotic-specific cyclin-B1 (CCNB1) and phosphorylated cyclin-dependent kinase 1 (CDK1), indicative of a G2/M cell cycle arrest (Fig. 2A). Flow cytometry analysis of propidium iodide-stained MDA-MB-231 cells confirmed that cells undergo a dose-dependent G2/M cell cycle arrest following MBZ treatment (Fig. 2B; Additional file 2: Fig. S2A, B). We also observed that MBZ treatment increased the expression of the apoptotic marker, cleaved PARP (poly ADP-ribose polymerase), and DNA damage marker, phosphorylated gamma-H2A histone family member X (γH2AX) (Fig. 2C). Likewise, there was a six-fold increase in dead cells following treatment with DMSO or 1 μM MBZ (Additional file 2: Fig. S2C). Altogether, the results demonstrated that, like other cancer types, MBZ induces G2/M cell cycle arrest in TNBC cells following 48 h of treatment and apoptosis after 72 h of treatment.
In vitro, MBZ reduces human TNBC cell migration
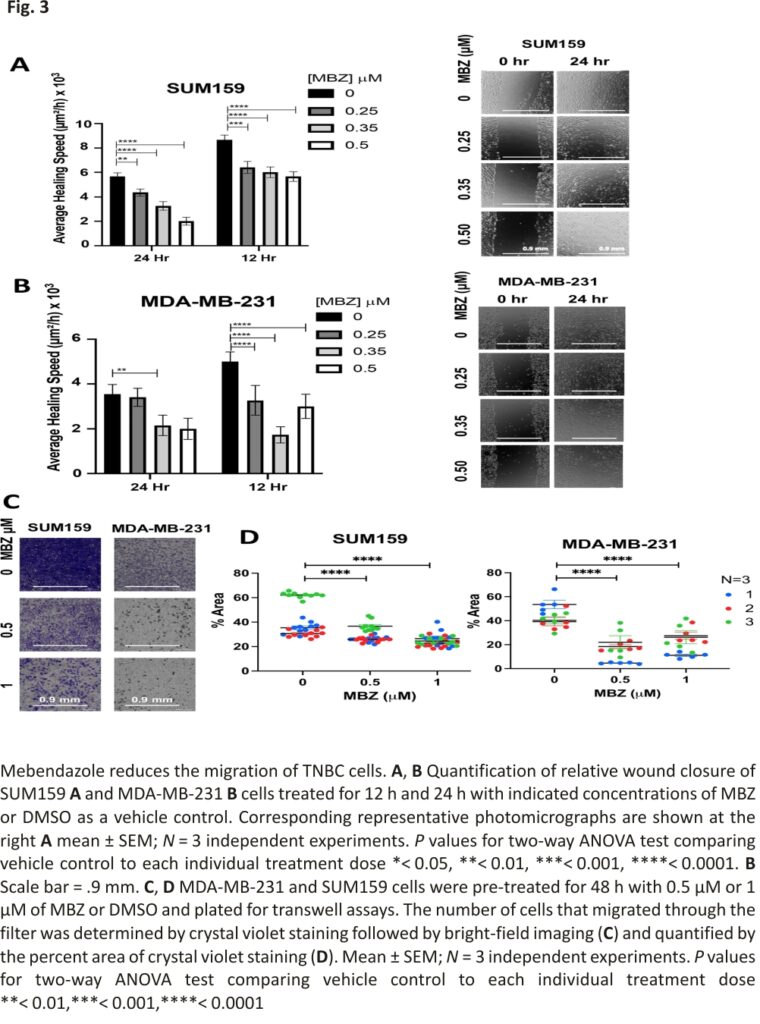
In addition, MBZ has been shown to reduce the migration and invasion of thyroid and gastric cancer cells 16,28 and more recently in MDA-MB-231 cells 29. Therefore, we used two independent assays to determine whether MBZ could also inhibit the migration of TNBC cells. First, we performed a wound-healing assay to assess the ability of MBZ to inhibit cell migration in vitro. SUM159 and MDA-MB-231 cells were grown to confluence, and a single scratch (or wound) was generated (0 h). The cells were treated with MBZ at a concentration of 0.25 μM, 0.35 μM, 0.5 μM, or with DMSO as vehicle control. The wounds were imaged at times 0 h, 12 h, and 24 h to calculate the average area of the wound covered with cancer cells. Compared to the vehicle control, MBZ treatment significantly decreased the average healing speed of the wound starting 12 h post-treatment with a 0.35 μM MBZ concentration in MDA-MB-231 cells (Fig. 3A, B). In a second migration assay, MBZ reduced the ability of SUM159 and MDA-MB-231 cells to migrate through Transwell pores following 48 h of pre-treatment with 0.5 μM and 1 μM of MBZ as compared to DMSO (Fig. 3C, D). Given the correlation between migratory ability and metastasis 36, our results suggest that even if a cell can survive MBZ treatment, it has a reduced capacity to migrate, and likely a decreased ability to metastasize.
MBZ reduces primary tumor growth and prevents distant organ metastases in an orthotopic model of TNBC
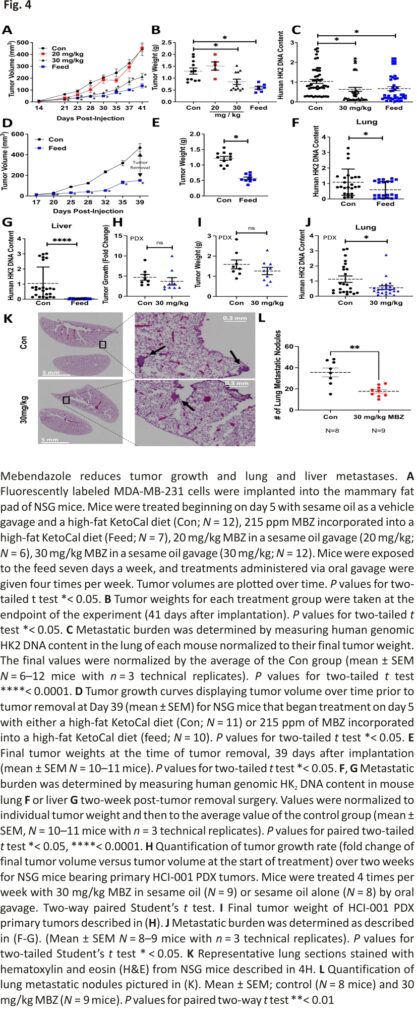
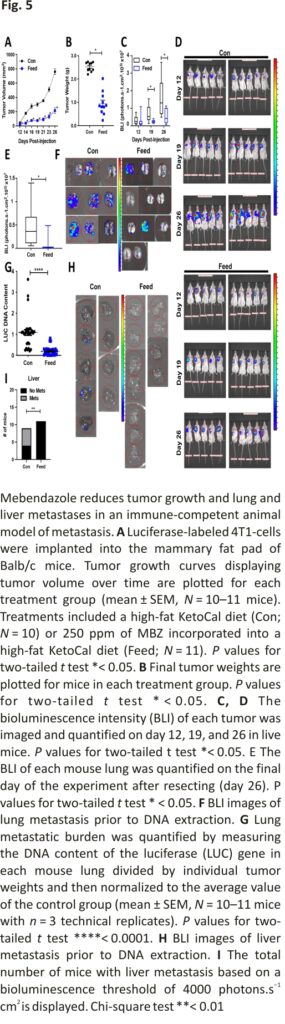
Previous studies have shown that orally administered MBZ reduced metastasis in orthotopic models of thyroid cancer 16 and adrenocortical carcinoma 37, respectively. Given these findings and the reduction in cell migration, we reasoned that MBZ would also reduce the metastatic spread of TNBC cells. We established orthotopic tumors by injecting fluorescently tagged MDA-MB-231 cells in the mammary fat pad of female NSG (NOD-SCID Gamma) mice. Five days post-injection, the mice were randomized and placed in the following treatment groups: sesame oil as a vehicle gavage and a high-fat KetoCal diet (Con; N = 12), 215 ppm MBZ incorporated into a high-fat KetoCal diet (Feed; N = 7), 20 mg/kg MBZ in a sesame oil gavage (20 mg/kg; N = 6; 4 times per week), or 30 mg/kg MBZ in sesame oil gavage (30 mg/kg; N = 12; 4 times per week). Mice treated with either 215 ppm MBZ feed or 30 mg/kg MBZ sesame oil gavage had slower tumor growth and 35% or 55% smaller primary tumors at the endpoint of the study as compared to control mice, respectively (Fig. 4A, B; Additional file 2: Fig. S3A, B). MBZ decreased metastatic burden by 60% and 72% as measured by quantifying the amount of human DNA content in mouse lungs for both the 215 ppm MBZ fed group and the group treated with 30 mg/kg MBZ by oral gavage as compared to the control group, respectively (Fig. 4C). The results suggested that MBZ has an even more dramatic effect on metastasis than the reduction in tumor growth.
To more closely recapitulate clinical protocols used to treat patients with BC, mice received 215 ppm MBZ incorporated into their feed using a neoadjuvant treatment protocol. Five days after cell implantation, mice were placed on MBZ feed. Tumors were surgically resected 5 weeks post-implantation and adjuvant MBZ treatment continued for two additional weeks. No toxicities were associated with the MBZ feed or surgery throughout the experiment as assessed by mouse body weight (Additional file 2: Fig. S3C). Still, the MBZ-fed mice had slower tumor growth and significantly smaller primary tumors at the time of resection (Fig. 4D, E). At the endpoint of the experiment, we extracted genomic DNA from mouse lungs and livers to measure metastatic spread using qPCR to quantify human HK2 DNA content. Mice treated with 215 ppm MBZ feed had significantly reduced lung (Fig. 4F; Additional file 2: Fig. S3D) and no detectable liver metastases (Fig. 4G; Additional file 2: Fig. S3E). To rule out the effect of primary tumor size contributing to the difference in metastasis, we normalized HK2 DNA content by individual tumor weight and compared it to the mean of the control group. The results demonstrated that MBZ reduces lung metastasis to a greater extent than it inhibits tumor growth and MBZ completely abolishes liver metastases.
We expanded our preclinical models by orthotopically implanting a TNBC patient-derived xenograft (PDX), HCI-001, in NSG mice. Following 8 weeks of growth, the primary tumors reached an average tumor volume of ~ 150 mm3 (Additional file 2: Fig. S3F, G). The mice were then randomized into two treatment groups which were dosed 4 times per week: sesame oil as a vehicle gavage (Con, N = 8) or 30 mg/kg MBZ in sesame oil gavage (30 mg/kg; N = 9). After two weeks of treatment, there was no decrease in the size of tumors for the MBZ group nor a reduction (Fig. 4H; Additional file 2: Fig. S3H, I) of tumor weight (Fig. 4I). However, we did observe a twofold decrease in metastatic burden in the lung (Fig. 4J). We further confirmed a significant decrease in lung metastases by counting the number of nodules present in H&E-stained mouse lungs. We observed a twofold reduction in lung nodules in mice treated with 30 mg/kg MBZ compared to mice given vehicle control (Fig. 4K, L; Additional file 2: Fig. S3J). The results highlight the ability of MBZ to reduce lung metastases in a TNBC PDX model, which has clinically relevant implications since PDX models closely match patients’ therapeutic responses. 38
MBZ decreases primary tumor growth and diminishes distant organ metastases in a syngeneic model of TNBC
Next, we tested the ability of MBZ to inhibit growth and metastasis of the 4T1 BC cell line that was derived from the mammary gland tumor of an immune-competent, BALB/c mouse. 4T1-luciferase-tagged cells were orthotopically implanted into the mammary fat pad of mice. MBZ decreased primary tumor growth. The final tumor weight was decreased by 2.6-fold in MBZ-fed mice compared to the control mice without toxicity (Fig. 5A, B; Additional file 2: Fig. S4A, B). In addition, in vivo bioluminescence images taken at several time points throughout the experiment illustrated that the tumors in MBZ-fed mice had decreased bioluminescence, indicating that they grew at a slower rate (Fig. 5C, D; Additional file 2: Fig. S4C). At the endpoint of the experiment, MBZ reduced lung bioluminescence by 6.5-fold compared to the control mice (Fig. 5E, F). To confirm our bioluminescence results, we also measured the luciferase DNA content in the lungs (Fig. 5G). Strikingly, bioluminescence could not be detected in the liver of MBZ-fed mice, confirming our previous observations (Fig. 5H, I). Overall in three in vivo mouse models of TNBC, MBZ reduced primary tumor growth by 2–threefold, lung metastasis by 2–sixfold (in four in vivo models), and completely eliminated liver metastasis.
MBZ reduces the cancer stem cell regulator, Integrinβ4, in TNBC cell lines
To uncover the main gene expression changes altered by MBZ treatment, we performed RNA sequencing of MDA-MB-231 and SUM159 cells following 72 h treatment of 1 μM MBZ compared to DMSO vehicle control. A total of 420 genes were upregulated (FC ≥ 1.5), and 140 genes were downregulated (FC ≤ 1.5) by MBZ treatment compared with cells treated with DMSO in both cell lines (Fig. 6A). Using a gene ontology (GO) enrichment analysis, we confirmed MBZ downregulated pathways that were involved in angiogenesis in TNBC cells as has been previously reported for medulloblastoma, colon cancer, and thyroid cancer (Additional file 2: Fig. S5A) 16,23,39. A principal component analysis showed that the trajectory of gene expression changes along PC3 was the same for both MDA-MB-231 and SUM159 cells following treatment with MBZ (Additional file 2: Fig. S5B).
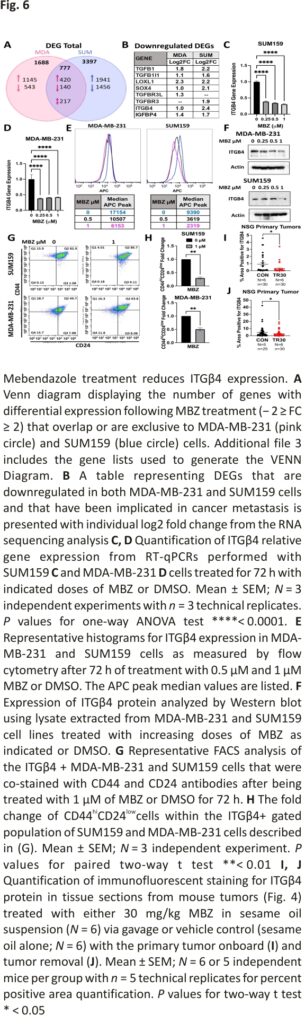
Due to the dramatic reduction in distant organ metastases in our in vivo TNBC mouse models, we focused on the most differential expression genes that were downregulated with MBZ treatment and that have also been previously reported to prevent or decrease BC metastases in animal models (Fig. 6B). We uncovered four well-known drivers of metastases whose expression was reduced following MBZ treatment: members of the transforming growth factor β (TGFβ) family, SRY-related HMG box 4 (SOX4), lysyl oxidase-like 1 (LOXL1), and Integrin β4 (ITGβ4).
We used RT-qPCR to confirm the ability of MBZ treatment to reduce the expression of the candidate genes (Fig. 6B). Only ITGβ4 gene expression was decreased in both cell lines following 72 h of MBZ treatment (Fig. 6C, D). To confirm this result at the protein level, ITGβ4 was measured in cells by flow cytometry analysis and Western blot analysis (Fig. 6E, F; Additional file 2: Fig. S5C, D). This reduction occurred dose-dependently beginning at concentrations as low as 0.05 μM in MDA-MB-231 cells and 0.125 μM in SUM159 cells (Additional file 2: Fig. 5C). In addition, MDA-MB-231 cells that survived 0.5 μM of MBZ (Sytox negative) had a 19-fold increase in the ITGβ4-negative population (Additional file 2: Fig. S5E).
Previously, ITGβ4 has been shown to be a marker of cancer stemness 40. To determine whether MBZ treatment reduced the ITGβ4-positive TNBC population in MDA-MB-231 and SUM159 cells and reduced the population of cells expressing conventional cancer stem cell markers (CSC) (CD44hiCD24low), we analyzed the percent of CD44hiCD24low cells within the ITGβ4-positive population. We determined that MBZ reduced cancer stem cell population by almost twofold in MDA-MB-231 cells and fourfold in SUM149 cells following 72 h of 1 μM MBZ treatment (Fig. 6G, H).
Next, we aimed to determine whether ITGβ4 expression was affected in tumors from MBZ-treated mice. Using RNA from the PDX tumors from mice treated as described in Fig. 4H, we determined that MBZ treatment led to a twofold decrease in ITGβ4 RNA expression compared to vehicle control-treated mice (Additional file 2: Fig. S6F). Next, we immunofluorescently labeled ITGβ4 protein in primary tumors of mice bearing MDA-MB-231 tumors and treated them as described in Fig. 4A, D. ITGβ4 was reduced by threefold (Fig. 6I; model first described in Fig. 4A) or twofold (Fig. 6J; model first described in Fig. 4D) in NSG mice treated with MBZ compared to vehicle control (Additional file 2: Fig. S5G). Overall, MBZ treatment in vitro and in vivo consistently reduced ITGβ4 RNA and protein expression.
MBZ treatment reduces cancer stem cell phenotype of TNBC cells
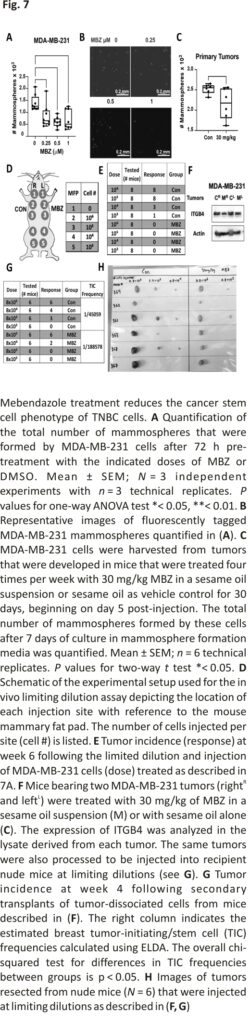
Previously, Zhang et al. 17 determined MBZ treatment in vitro reduced the number of mammospheres formed by SUM159 and MDA-MB-231 cells treated with MBZ versus DMSO. Similarly, we found that MBZ reduced the mammosphere formation of MDA-MB-231 cells by 40% when treated with 0.25 μM MBZ compared to DMSO (Fig. 7A, B; Additional file 2: Fig.S6A). We also demonstrated that ITGβ4+ tumor-derived MDA-MB-231 cells formed 4× more mammospheres than ITGβ4-cells (Additional file 2: Fig. S6B), supporting its role as a stem cell marker as previously shown 40. We observed a 15% reduction in the ability of tumor-derived MDA-MB-231 cells from NSG mice treated for 30 days with MBZ to form mammospheres compared to tumor-derived cells from a vehicle control-treated mouse (Fig. 7C; Additional file 2: Fig. S6C).
Next, we utilized a limiting dilution assay to evaluate the ability of MDA-MB-231 cells treated with mebendazole in vitro or in vivo to form tumors in nude mice (Fig. 7D). MDA-MB-231 cells were pre-treated in vitro for 72 h with DMSO alone or treated with 1 μM MBZ. After treatment, the viable cells were injected at limiting dilutions into nude mice. DMSO-treated cells had a tumor-initiating cell (TIC) frequency of 1/17,169 cells, whereas cells treated with MBZ did not develop tumors at any dilution (Fig. 7E; Additional file 2: Fig. S6D–G). The drastic reduction in self-renewal capabilities in cells pre-treated with MBZ in vitro prompted a second limiting dilution assay (LDA). NSG mice bearing MDA-MB-231 cell-derived tumors were treated for 30 days with 30 mg/kg MBZ or sesame oil alone four times per week. As previously shown, MBZ treatment reduced tumor growth, ITGβ4 expression, and lung metastasis (Fig. 7F; Additional file 2: Fig. S6H–J). Likewise, the tumor-derived MDA-MB-231 cells treated for 30 days with a vehicle control were more efficient at tumor initiation, with a TIC frequency of 1/45,059 compared to the TIC frequency of 1/188,578 from tumor-derived MDA-MB-231 cells treated with MBZ (Fig. 7G). The fourfold difference in TIC frequency corresponded with decreased tumor burden at each injection site (Fig. 7H; Additional file 2: Fig. S6K–M). The data suggested that cells that survive MBZ treatment in the primary tumor have reduced ITGβ4 expression and a decreased ability to form mammospheres and initiate tumor formation.
Discussion
Our preclinical studies demonstrated that the anthelminthic, MBZ, decreased the growth of TNBC and significantly abrogated lung metastasis while eliminating liver metastases. We showed that MBZ decreased TNBC cell proliferation through previously described mechanisms of action, including G2/M cell cycle arrest and apoptosis 16. Using RNA sequencing, we discovered that MBZ also reduces ITGβ4 expression and previous studies have linked ITGβ4 expression with increased metastasis 41. Likewise, targeting ITGβ4 with ITGβ4 protein-pulsed dendritic cell vaccination or via an anti-CD3/anti-ITGβ4 bispecific antibody decreased metastasis in mouse models of BC 42. ITGβ4 can be used to stratify TNBC cells that differ in their tumor initiation abilities and has been used as a cancer stem cell marker to identify more aggressive subtypes of TNBC 40. Our studies show that MBZ decreases ITGβ4 expression concomitant with decreases in cell stemness in vitro and in vivo as measured by mammosphere formation and limiting dilution assays. Taken together, the results suggest that a reduction in ITGβ4 expression may contribute to the effectiveness of MBZ in reducing metastasis.
A recent high throughput screening study identified MBZ as a therapeutic that could inhibit radiation-induced de-differentiation in the TNBC cell line, SUM159. In this study, MBZ had impressive in vitro efficacy but only a modest in vivo efficacy (10 mg/kg or 20 mg/kg I.P. for 3 weeks) when used as a single agent 17. In our study, we showed that MBZ decreased TNBC cell proliferation and viability using human (SUM159, MDA-MB-231) and mouse (4T1, MMTV-PyMT) BC cell lines. We show that MBZ has significantly greater therapeutic efficacy than in the Zhang et al. study. This may be due in part to differences in the method of delivery of MBZ. We delivered MBZ polymorph C orally by incorporating it into a high-fat diet or using a high-fat suspension such as sesame oil. Previous studies have shown that this delivery method increased the absorption of MBZ. Additionally, Polymorph C has higher absorption than other polymorphs that have been tested in mouse models 16,19,20.
TNBC is the most challenging BC subtype to treat and results in higher mortality due to the high incidence of metastatic cases and the paucity of treatment options. Most patients with BC would survive their disease if metastasis could be prevented. Our preclinical results showed that MBZ reduced metastasis with no side effects in mice, and it is well tolerated in humans even at higher doses and for longer durations than used in mice 27. Given this safety profile, MBZ is a candidate for long-term use and may have promise as adjuvant therapy following surgical resection to prevent BC metastasis. Future studies considering the addition of MBZ to the standard of care treatment are warranted in order to advance MBZ in clinical trials for patients with breast cancer.
Availability of data and materials
The data generated in this study are available in Gene Expression Omnibus (GEO) at GSE190845.
References
1. Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71: 209–49.
2. Sørlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40: 2667–75.
3. Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–52.
4. Sørlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869 –74.
5. Brenton JD, Carey LA, Ahmed A, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–60.
6. Taherian-Fard A, Srihari S, Ragan MA. Breast cancer classification: linking molecular mechanisms to disease prognosis. Brief Bioinform. 2014;16: 461–74.
7. Harbeck N, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:1–31.
8. Newton EE, Mueller LE, Treadwell SM, Morris CA, Machado HL. Molecular targets of triple-negative breast cancer: where do we stand? Cancers (Basel). 2022. https://doi.org/10. 3390/cancers 14030482.
9. Wang R, et al. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019; 19:1091.
10. Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109 –12.
11. Miller MJ, Krupp IM, Little MD, Santos C. Mebendazole: an effective anthelmintic for trichuriasis and enterobiasis. JAMA. 1974;230: 1412– 4.
12. Guerini AE, et al. Mebendazole as a candidate for drug repurposing in oncology: an extensive review of current literature. Cancers (Basel). 2019. https://doi.org/10.3390/ cancers11091 284.
13. Williamson T, et al. Mebendazole disrupts stromal desmoplasia and tumorigenesis in two models of pancreatic cancer. Oncotarget. 2021; 12:1326–38.
14. Sasaki J-I, et al. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Molecular Cancer Ther. 2002; 1(13): 1201–9.
15. Mukhopadhyay T, Sasaki JI, Ramesh R, Roth JA. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2002;8:2963–9.
16. Williamson T, Mendes TB, Joe N, Cerutti JM, Riggins GJ. Mebendazole inhibits tumor growth and prevents lung metastasis in models of advanced thyroid cancer. Endocr Relat Cancer. 2020;27:1–5.
17. Zhang L, et al. Mebendazole potentiates radiation therapy in triple-negative breast cancer. Int J Radiat Oncol Biol Phys. 2019; 103: 195–207.
18. Skibinski CG, Williamson T, Riggins GJ. Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma. J Neurooncol. 2018;140: 529–38.
19. Bai RY, et al. Brain penetration, and efficacy of different mebendazole polymorphs in a mouse brain tumor model. Clin Cancer Res. 2015;21: 3462–70.
20. Bai R-Y, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. Antiparasitic mebendazole shows survival benefits in 2 preclinical models of glioblastoma multiforme. Neuro Oncol. 2011. https://doi.org/10. 1093/neuonc/nor077.
21. Bai R-Y, Staedtke V, Rudin CM, Bunz F, Riggins GJ. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol. 2015. https://doi.org/10.1093/ neuonc/nou23
22. Simbulan-Rosenthal CM, et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61K melanoma. Oncotarget. 2017;8: 12576–95.
23. Williamson T, Bai RY, Staedtke V, Huso D, Riggins GJ. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget. 2016;7: 68571–84.
24. Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—mebendazole as an anti-cancer agent. Ecancermedical science. 2014. https://doi.org/10. 3332/ecancer. 2014.443.
25. Doudican N, Rodriguez A, Osman I, Orlow SJ. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res.2008; 6: 1308–15.
26. Petersen JSSM, Baird SK. Treatment of breast and colon cancer cell lines with anti-helminthic benzimidazoles mebendazole or albendazole results in selective apoptotic cell death. J Cancer Res Clin Oncol. 2021;147: 2945–53.
27. Gallia GL, et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of phase 1 clinical trial. Neuro-Oncology Adv. 2021;3:1–8.
28. Pinto LC, et al. The anthelmintic drug mebendazole inhibits growth, migration, and invasion in gastric cancer cell models. Toxicol Vitro. 2015;29:2038 –44.
29. Choi HS, et al. molecules anticancer effect of benzimidazole derivatives, especially mebendazole, on triple-negative breast cancer (TNBC) and radiotherapy-resistant TNBC in vivo and in vitro. Molecules. 2021. https://doi.org/10.3390/molecules 26175118.
30. Zhou J, et al. Stem cells and cellular origins of breast cancer: updates in the rationale, controversies, and therapeutic implications. Front Oncol. 2019;9:820.
31. Godet I, et al. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun. 2019;10: 4862.
32. Bhoyar S, Godet I, DiGiacomo JW, Gilkes DM. A software tool for the quantification of metastatic colony growth dynamics and size distributions in vitro and in vivo. PLoS ONE.2018; 13: e0209591.
33. DeRose YS, et al. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol. 2013;60:14–23.
34. Godet I, Mamo M, Thurnheer A, Rosen DM, Gilkes DM. Post-hypoxic cells promote metastatic recurrence after chemotherapy treatment in TNBC. Cancers (Basel). 2021; 13:5509.
35. Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–8.
36. Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer. 2016;17:131–40.
37. Martarelli D, Pompei P, Baldi C, Mazzoni G. Mebendazole inhibits the growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother Pharmacol. 2007. https://doi. org/10. 1007/s00280-007-0538-0.
38. Byrne AT, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–68.
39. Larsen AR, et al. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 2015;14:3–13.
40. Bierie B, et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci.2017. https://doi.org/10.1073/pnas. 1618298114.
41. Li XL, et al. Integrin β4 promotes cell invasion and epithelial-mesenchymal transition through the modulation of Slug expression in hepatocellular carcinoma. Sci Rep 71. 2017;7(1):1–1 2.
42. Dobson HE, Ruan S, Chang AE, Wicha MS, Li Q. Targeting cancer stem cells via integrin β4. Oncotarget. 2021;12:1850.
Acknowledgments
We thank Dr. Sara Sukumar, Dr. Jenni Sheng, and Dr. Cynthia Zahnow for providing helpful advice for the project and clinical insight.
Funding
Work in the Gilkes lab is supported by The Jayne Koskinas Ted Giovanis Foundation for Health and Policy, The Emerson Collective, and The Allegany Health Network. Work in the Riggins lab was supported by the Virginia & DK Ludwig Fund for Cancer Research.
Author information
Authors and Affiliations
Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, 21231, USA
Natalie S. Joe, Inês Godet, Harsh H. Oza, Gregory J. Riggins & Daniele M. Gilkes
Cellular and Molecular Medicine Program, The Johns Hopkins University School of Medicine, Baltimore, MD, 21231, USA
Natalie S. Joe, Gregory J. Riggins & Daniele M. Gilkes
Department of Chemical and Biomolecular Engineering, The Johns Hopkins University, Baltimore, MD, 21218, USA
Inês Godet, Nubaira Milki & Daniele M. Gilkes
Johns Hopkins Institute for NanoBioTechnology, The Johns Hopkins University, Baltimore, MD, 21218, USA
Inês Godet & Daniele M. Gilkes
NIH NIDDK Short-Term Research Experience Program to Unlock Potential (STEP-UP), The Johns Hopkins University School of Medicine, Baltimore, MD, 21231, USA
Noor U. I. Ain
Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD, 21231, USA
Gregory J. Riggins
Contributions
NSJ, GJR, and DMG contributed to the conception and design, and development of the methodology. NSJ, IG, NM, NUA, and HHO were involved in the acquisition of data (provided animals, acquired and manages patients, provided facilities, etc.). NSJ, IG, NM, NUA, and DMG contributed to the analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis). NSJ and DMG were involved in writing, reviewing, and/or revision of manuscripts and administrative, technical, or material support (i.e., reporting or organizing data, and constructing databases). DMG contributed to study supervision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Daniele M. Gilkes.
Ethics declarations
Ethics approval and consent to participate
All animal research complied with relevant ethical regulations within protocols approved by the Johns Hopkins University Animal Care and Use Committee.
Competing interests
The authors have no competing interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Credits: Joe, N.S., Godet, I., Milki, N. et al. Mebendazole prevents distant organ metastases in part by decreasing ITGβ4 expression and cancer stemness. Breast Cancer Res 24, 98 (2022). https://doi.org/10. 1186/s13058-022-01591-3