Elisabetta Sannaa, Clelia Madeddub, Fabrizio Lavraa, Sara Oppic, Mario Scartozzib, Pier Giorgio Calòd, Antonio Macciòad
aDepartment of Gynecologic Oncology, A. Businco Hospital, ARNAS G. Brotzu, 09100, Cagliari, Italy
bMedical Oncology, Azienda Ospedaliero Universitaria di Cagliari, Department of Medical Sciences and Public Health, University of Cagliari, 09100, Cagliari, Italy
cHematology and Transplant Center, A. Businco Hospital, ARNAS G. Brotzu, 09100, Cagliari, Italy
dDepartment of Surgical Sciences, University of Cagliari, 09100, Cagliari, Italy
Highlights
• Isolated lymph node recurrence (ILNR) in gynecological cancers is uncommon.
• Data on the feasibility and effectiveness of minimally invasive surgery for ILNRs are limited.
• We performed successful laparoscopic lymphadenectomy for ILNRs from gynecological cancers.
• Minimally invasive surgery was effective even for large nodes up to 8 cm.
• Laparoscopic surgery also allows early initiation of postoperative systemic chemotherapy.
Abstract
Background
The surgical treatment of isolated lymph node recurrence (ILNR) of gynecological malignancies is still debated. The feasibility and effectiveness of minimally invasive lymphadenectomy have been reported by few studies; however, it remains unclear what the upper tumor size limit is for a minimally invasive approach. We prospectively analyzed cases of ILNR treated by laparoscopy in our unit while focusing on the safety and feasibility of resecting large tumors suspected of recurrence using a minimally invasive approach.
Materials and methods
We carried out a prospective observational case-series study. We included all consecutive patients with ILNR from gynecological cancers who underwent minimally invasive lymphadenectomy at our unit from June 2013 to June 2021 to assess the safety and feasibility of such a surgical approach. We also evaluated the oncological outcome in terms of further recurrence, site of recurrence, and survival.
Results
Twenty-seven patients with ILNR due to gynecological malignancies were included (ovarian cancer, 12; uterine malignancies, 12; cervical cancer, 3). Three had remarkably large LNs up to 8 cm: these emblematic cases have been reported in detail with accompanying videos of the surgical procedure. The most frequent site of ILNR was aortic (67%). Recurrent LNs were completely resected in all cases; none of the procedures was converted to open surgery. The median follow-up duration was 24 months. Ten patients (37%) had a new recurrence. To date five patients (18.5%) have succumbed, four (14.8%) are alive with evidence of disease, and 18 (66.7%) are alive with no evidence of disease.
Conclusions
Minimally invasive surgery for ILNR in gynecological malignancies may be an option feasible, safe, and effective in terms of oncological outcomes, even for large tumors. It also allows quicker recovery with early initiation of appropriate postoperative systemic chemotherapy, in the context of an optimal multimodal therapeutic approach.
Keywords
Isolated nodal recurrence; Gynecological cancers; Minimally invasive surgery; Laparoscopy; Secondary cytoreductive surgery; Disease-free survival; Case-series
1. Introduction
Lymph node (LN) involvement occurs frequently in gynecological malignancies; however, isolated LN recurrence (ILNR) is uncommon, and studies on this condition have been limited 1,2,3. ILNRs occur as a consequence of an initial refuge for microscopic disease and are a common failure site in pre-treated patients 4. The most frequent anatomic sites for ILNR, which occur in approximately 2%–4% of patients, are the pelvic and para-aortic/para-caval regions 5,6.
Currently, surgery is the recommended treatment for ILNRs, especially when the recurrence is in an isolated LN 7. Although concurrent chemo-radiotherapy (CCRT) has proven to be a successful salvage treatment for ILNRs, some studies have demonstrated the advantages of surgery in terms of improved survival rate and better local control in comparison to radiotherapy or chemotherapy alone 6,7,8.
In this context, minimally invasive surgery could have a specific and important role. Schlaerth et al. 9 demonstrated the benefits of minimally invasive surgery for gynecological cancers, emphasizing its role in facilitating accurate pretreatment staging, the possibility of primary debulking (including LN debulking), and in guiding further individualized/tailored treatment (radiotherapy, chemotherapy, and chemo-radiotherapy). They also highlighted the role of operative laparoscopy as a valuable step in the workup of locally advanced cancers, which could help determine the eligibility for further surgeries and avoid unnecessary laparotomies. La Verde et al. 10 also illustrated the strengths of ultra-minimally invasive surgery in terms of superior cosmetic outcomes, pain relief, less bleeding and transfusion requirements, less morbidity, shorter postoperative hospital stay, better quality of life, and excellent surgical outcomes; however, they also indicated the main weaknesses of this procedure, which included potential coagulation damage and risks related to limitations in tissue manipulation, particularly the vascular structures. The advantages described for minimally invasive surgery also apply to the use of robot-assisted laparoscopy in gynecological surgery in terms of perioperative outcomes, i.e., reduced operating time, reduced blood loss, decreased pain, quicker return to a normal diet and early ambulation, shorter hospital stay, and similar intra- and postoperative complications and long-term oncological outcomes (disease-free and overall survival) in comparison to laparotomies 11,12, 13. All these endpoints are likely to be equally met in laparoscopic surgery for ILNR. Moreover, laparoscopic minimally invasive surgeries for ILNRs could allow a rapid and accurate histological diagnosis of relapse, allowing early initiation of the most appropriate and specific systemic treatment.
Few studies have reported on the feasibility and effectiveness of minimally invasive lymphadenectomy for ILNR treatment 14,15,16,17,18,19. Additionally, the ideal candidates for laparoscopic lymphadenectomy have not been defined. Identifying prognostic factors associated with survival is needed to accurately assess the treatment outcome. Despite the well-established benefits of laparoscopic surgery 14, performing laparoscopic lymphadene- ctomy for a large metastatic LN can be difficult due to the limited accessibility during surgery. Thus, laparoscopy for large ILNRs remains controversial. Moreover, para-aortic and para-caval lymphadenectomy is technically difficult and has steep learning curves 20. Further, ILNR is distinguished as being larger and more tightly adherent; hence, resection is difficult even through laparotomy. In addition, in cases of para-aortic recurrences, identifying whether the disease extends above the inferior mesenteric artery or is limited within the retrocaval or retroaortic basin, along with knowledge of the anatomical relationships with the ureters is essential. Another indefinite factor for laparoscopic surgery for ILNR is the upper threshold of the tumor size.
In this study, we prospectively analyzed cases of ILNR treated by laparoscopy in our unit while focusing on the safety and feasibility of resecting large tumors suspected of recurrence using a minimally invasive approach.
2. Materials and Methods
This study is reported in line with the PROCESS 2020 criteria 21. We carried out a case series prospective single-center observational study including all patients who consecutively underwent minimally invasive lymphadenectomy for ILNR associated with gynecological cancers at a Tertiary Referral University Hospital Gynecologic Oncology Unit from June 2013 to June 2021. The study was conducted in accordance with the Declaration of Helsinki. The protocol was notified and approved by the Local Institutional Review Board on 15 November 2021, in accordance with the National Regulatory Agency for observational trials not involving drugs. Each patient provided written informed consent for the surgical and medical treatment as well as for data collection and analysis for scientific purposes. The study has been registered at https://www.research registry.com with the unique identifier registration number “research registry7754”; https://www.research registry.com/registernow#home/registrationdetails/6238cda220963a001edd451d/. Prospective anonymized data regarding all consecutive surgeries performed were collected and retained in a secure database.
The inclusion criteria comprised: patients who were eligible for laparoscopic lymphadenectomy for suspected ILNR diagnosed during follow-up examinations by computed tomography (CT) and whole-body positron emission tomography (PET)/CT after primary surgery for cervical, ovarian, primary peritoneal, or uterine cancer; patients with good clinical performance status (Eastern Cooperative Oncologic Group performance status 0–2); stable medical condition; and absence of comorbidities that contraindicate laparoscopy. The exclusion criteria comprised: evidence of extra-abdominal disease identified by imaging exams or disseminated peritoneal carcinomatosis verified during laparoscopic abdominal assessment; the presence of ascites; high anesthesiologic risk (American Society of Anesthesiologists Physical Status ≥ III); the presence of any preoperative medical contraindication to laparoscopy (such as reduction in the respiratory capacity, inability to tolerate Trendelenburg position or pneumoperitoneum throughout the entire surgical procedure); or intraoperative causes impeding minimally invasive surgical procedures. History of prior abdominal surgeries or high body mass index (BMI) did not preclude surgery.
The patients’ demographics and anthropometric characteristics, including information regarding tumor histology and the International Federation of Gynecology and Obstetrics (FIGO) stage on initial diagnosis, history of prior surgeries, operative time, estimated blood loss (EBL), the number of days of postoperative hospital stay, comorbidities, and intra-operative and post-operative complications within 30 days from surgery were collected from the medical records. Operating time was calculated as the time taken from performing the first incision to the final suture at all port sites.
As part of pre-operative planning, all patients underwent pre-operative imaging examinations (CT and PET/ CT) to assess the disease extent and location, and its anatomical relationships with the peritoneal and retroperitoneal structures for possible involvement of the ureters, intestines, nerves, and vessels. The patient with the largest LN mass also had a consultation with the hematologist.
Post-operative complications included those appearing up to 8 weeks post-operatively; they were scored according to the Clavien– Dindo classification 22. Post-operative evaluations with daily clinical assessments were performed; fibrinogen levels were monitored as an early diagnostic marker of complications 23. After discharge, the patients were checked by phone call every 2 days. They visited the clinic for a follow-up at 1 week and then every 4 weeks or sooner if symptoms were observed; this continued for 2 months post-operatively. Long-term follow-up outcomes and further recurrences were recorded; during follow-up, the patients underwent laboratory analyses including specific tumor marker tests every 3 months, and PET/CT or CT every 4–6 months.
2.1. Surgical techniques
Throughout the study duration, all surgeries were performed by a single experienced gynecological surgery team, where the senior principal surgeon was a clinician, who specialized in Obstetrics and Gynecology and Medical Oncology, with extensive training and experience in both gynecologic oncology and minimally invasive surgery of the pelvis and upper abdomen with more than 4500 major surgical procedures, including more than 1600 surgeries for gynecological cancers (about 70% performed laparoscopically).
Perioperative antibiotic therapy and postoperative thromboembolic prophylaxis were used routinely. All surgeries were performed with the patients intubated and under general anesthesia. In consideration of the high surgical risk, specifically vascular injuries, all laparoscopic tools (grasper, vascular clips, clamps, bipolar instruments) were prepared in the operating room to manage potential complications. Furthermore, a laparotomy set was always kept ready for conversion to open surgery in case of severe injury or acute bleeding. Additionally, blood units for transfusion were available before surgery was started. We performed pelvic and para-aortic lymphadenectomy using a variable number of trocar access: one large 12-mm trocar was positioned at the umbilicus; one 12-mm trocar was placed laterally midway between the umbilicus and the right anterior superior iliac crest; one 5-mm trocar was inserted at the midline near the symphysis pubis, and one 5-mm trocar was inserted laterally midway between the umbilicus and the left anterior superior iliac crest; further ancillary trocars were positioned at Palmer’s point on the left or at a corresponding point on the right, depending on the surgical need. The patient was placed in a Trendelenburg position. During pelvic lymphadenectomy, the first assistant stood on the right side of the patient while the surgeon was positioned on the left side of the patient with a view of the monitor. For an ergonomic working angle, the trocar at Palmer’s point or its equivalent on the right was positioned almost parallel to the surgical axis. During para-aortic and para-caval lymphadenectomy, the surgeon mainly stood between the patient’s legs and viewed a monitor placed above the patient’s head; the first assistant stood on the left side of the patient and viewed the same monitor. Adhesiolysis was performed if required. We have performed pelvic and para-aortic/para-caval lymphadenectomy using a transperitoneal technique 20. In case of very large metastatic recurrences, the resection was started with the incision above the peritoneum (Supplementary Videos 1–2).
LN dissection was performed using an ultrasonic dissector (Harmonic Scalpel, Ultracision, Ethicon Endosurgery Inc., Cincinnati OH) or LigaSure Maryland Jaw Laparoscopic Sealer/Divider (Covidien, Boulder, CO) or LigaSure Blunt Tip Laparoscopic Sealer/Divider (Covidien, Boulder, CO). Hemostasis was obtained with BiClamp® LAP and BiClamp® LAP Maryland Forceps (Erbe, Germany).
For hemostasis and dissection, 5-mm Endo Peanut Blunt Dissector (Covidien, Boulder, CO), 10-mm Endopath blunt Cherry Dissector (Ethicon, Hamburg, Germany), and gauzes were used (Supplementary Videos 1,2) 17. A laparoscopic fan retractor was used for a better view of the anatomical structures (Supplementary Video 1). To prevent port-site metastasis and to avoid potential tumor cell spillage, we introduced an appropriately sized endobag (Endocatch, Ethicon) to contain the specimen. The endobag was then extracted either via the 12-mm port or an enlarged incision at another port site, depending on the specimen size. Antibiotic treatment was routinely continued for 3 or 4 days postoperatively.
2.2. Statistical analysis
We performed a descriptive analysis of the data. Results are presented as mean ± standard deviation for parametric data, and median (range) for non-parametric data. The interval from primary surgery to ILNR was calculated from the date of primary surgery to documentation of ILNR; follow-up duration from secondary cytoreductive surgery (SCS) for ILNR was calculated from the date of SCS to the last follow-up visit or the date of death. The SPSS statistical software program, version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
3. Results
Twenty-seven consecutive patients with ILNR due to gynecological malignancies were included. The patients’ clinical characteristics are reported in Table 1. The median age was 66 (range, 49–81) years, and the median BMI was 28 (range, 18–34). The primary diagnoses were 12 (44.4%) ovarian cancers, 12 (44.4%) uterine malignancies, and 3 (11.1%) cervical cancers. During the primary surgical treatment, 18 patients (66.6%) underwent lymphadenectomy. Fifteen (55.5%), 10 (37%), and two (7.5%) patients underwent radical surgery alone, radical surgery and adjuvant chemotherapy, and neoadjuvant chemotherapy and radical surgery, respectively. Eighteen (67%) and nine (34%) patients had LN recurrence in the aortic and pelvic regions, respectively.
The surgical findings are reported in Table 2. Pathological examination of all resected specimens confirmed LN metastases in all cases. The median number of resected pelvic LNs was 11.7 (range, 5–18) with a median of two metastatic LNs (range, 1–13). The median number of resected aortic LNs was 14.8 (range, 2–48) with a median of 5.6 metastatic LNs (range, 1–26). The maximum diameter of the largest resected nodal mass was 8 cm. Complete resection of recurrent LN was verified by post-operative imaging assessment in all cases.
The peri-operative and post-operative outcomes are shown in Table 3. None of the procedures required conversion to open surgery. No intra- and postoperative complications were observed. After lymphadenectomy, all patients underwent adjuvant therapy (chemotherapy, 12; radiotherapy, 3; chemoradiotherapy, 3). The median duration from surgery to initiation of adjuvant chemotherapy was 7 days (range, 5–10 days).
The median follow-up duration from secondary cytoreduction for ILNR was 24 months (range: 6–89 months). The follow-up outcomes are presented in Table 4. During this period, 10 patients (37%) had a new recurrence. After confirming new recurrence, all patients received systemic chemotherapy according to appropriate regimens indicated for the specific histotype. To date, five patients (18.5%) have succumbed to the disease, four patients (14.8%) are alive with evidence of disease, and 18 patients (66.7%) are alive with no evidence of disease.
3.1. Report of emblematic cases with remarkably large ILNR and accompanying videos
We have provided a detailed report of three emblematic cases with accompanying surgical videos, wherein the excised masses were remarkably large. The supplementary videos can be downloaded at https://zenodo. org/record/6060532#.YjoGeU2 ZM2w (DOI 10.5281/zenodo.60605 32).
3.1.1. Case 1
The patient was a 59-year-old woman with a history of ovarian high-grade serous carcinoma (HGSC). A follow-up PET/CT scan 36 months after primary surgery and adjuvant chemotherapy showed an increase in metabolic uptake at the aortocaval area, indicative of ILNR. Laparoscopy was performed, wherein a mass measuring 5.5 × 5.5 × 2.5 cm and strongly adhered to the right common iliac artery, vena cava, and right ureter was resected (Supplementary Video 1). The patient further experienced an LN recurrence at the pelvic and para-aortic LNs after 43 months of follow-up. She subsequently underwent laparoscopic lymphadenectomy followed by platinum-based chemotherapy. She is still alive.
3.1.2. Case 2
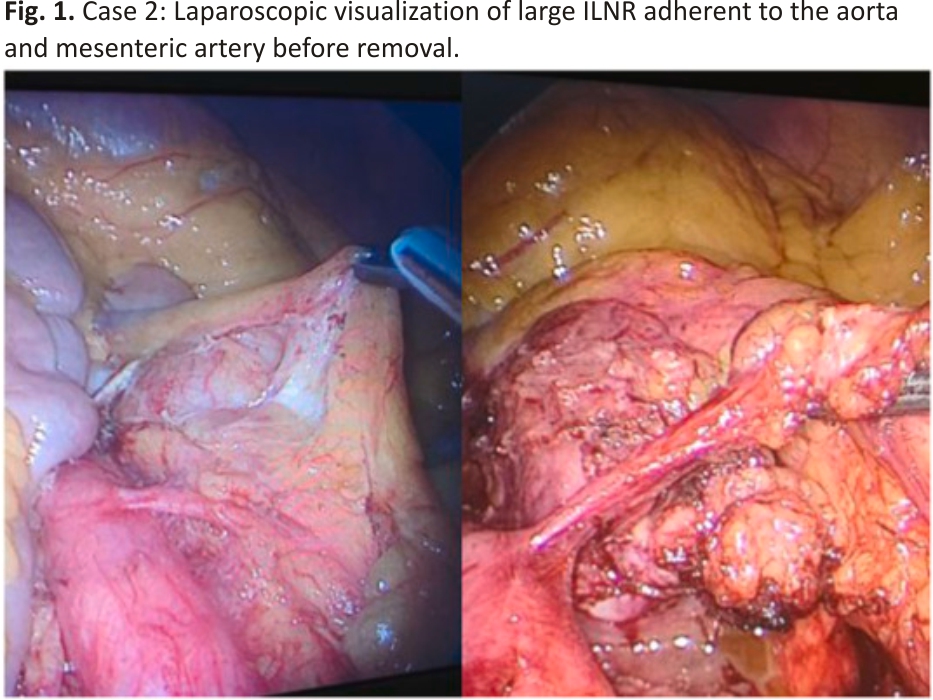
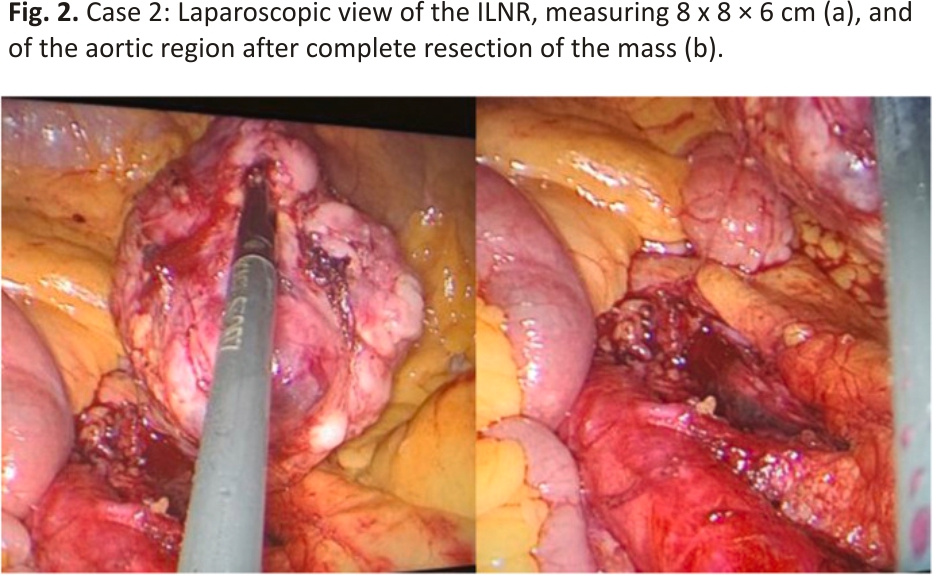
The patient was a 74-year-old woman with a history of ovarian HGSC. A follow-up PET/CT scan 29 months after primary surgery and adjuvant chemotherapy revealed increased metabolic uptake at the aortocaval area, suggestive of ILNR. Laparoscopy was performed, wherein a mass measuring 8 × 8 × 6 cm and strongly adhered to the aorta and mesenteric artery was resected (Fig. 1, Fig. 2).
The patient is alive after 32 months following surgery, with no evidence of disease at the follow-up examination.
3.1.3. Case 3
The patient was a 61-year-old woman with a history of uterine sarcoma. At a follow-up PET/CT scan 14 months after primary surgery, an increased metabolic uptake was reported in the pelvic area, which was suggestive of ILNR. We performed laparoscopic resection of a mass measuring about 4 × 3 × 3 cm, located between the obturator fossa, left ureter, and bladder (Supplementary Video 2). After 22 months from the SCS, the patient is still alive with no evidence of further recurrence.
4. Discussion
Several studies have emphasized the advantages of surgery in recurrent gynecological malignancies, especially when the relapse is isolated and the patient achieved a long disease-free survival after primary treatment 24,25. However, the surgical strategy for ILNR following primary treatment has not been established, which represents a clinical challenge for gynecological oncologists.
The aim of SCS is to prolong survival as well as improve the quality of life and control cancer-related symptoms. In terms of recurrence sites, nodal involvement is frequent, but ILNR is uncommon. Patients with ILNR can be defined as a selected group who can particularly benefit from SCS 2. A Cochrane review 26 analyzed the literature regarding the role of SCS for recurrent epithelial ovarian cancer (EOC); the authors compiled nine retrospective and prospective non-randomized studies; randomized clinical trials (RCT) were not available. In this report, the included studies showed that complete cytoreduction is related to a significant increase in overall survival in patients with platinum-sensitive recurrence. Due to the lack of data from RCTs, the authors assumed the results cannot be assuredly attributed to the surgical outcome or to tumor biology. Since salvage chemotherapy or radiotherapy of a bulky node can have a modest effect on EOC patients with ILNR, SCS may be subsequently performed. Furthermore, retroperitoneal nodal metastases seem to be more resistant to chemotherapy than other intraabdominal recurrences. This may be attributed to biological factors such as reduced vascularization and the resulting low availability of cytotoxic drugs 27. Survival after recurrence and overall survival were significantly increased in EOC patients with ILNR who underwent open SCS followed by chemotherapy compared to those who underwent chemotherapy alone 28. Fotiu et al. 29 reported a 5-year survival of 68% with a median follow-up of 45 months in 21 EOC patients following open SCS for ILNR. Uzan et al. 30 demonstrated a 5-year survival of 71% in 12 EOC patients who underwent SCS for ILNR. Ferrero et al. 2 reported a 64% 5-year overall survival in patients with recurrent EOC who underwent open SCS for ILNR with a median follow-up of 50 months.
While surgery followed by chemotherapy is considered the most appropriate approach for ILNR in EOC, the appropriate treatment approach for ILRN metastases in cervical cancer is more controversial. Salvage radiotherapy or chemo-radiotherapy is often performed to avoid the risk of not radical surgical resection and technical issues in gaining access and removing metastatic nodes 31. A major limitation in performing complete surgery, especially for inexperienced surgeons, occurs in the case of extracapsular invasion 32. However, surgical resection of ILNRs (+/− additional adjuvant therapy) results in progression-free survival and local control rate that are superimposable to CCRT and better than those achieved by radiotherapy or chemotherapy alone 33.
Similarly, the role of SCS in recurrent endometrial cancer remains debatable; a meta-analysis including 14 retrospective cohort studies showed its efficacy in selected patients who underwent an optimal cytoreduction 34. Consistently, relapsed endometrial cancer exhibiting an ILNR may be considered eligible for SCS.
Another concern regarding surgery for metastatic para-aortic LN is that it can be complicated by the damage of retroperitoneal vessels, particularly major veins such as the inferior vena cava (IVC) and the renal veins. Different procedures, including the tape traction maneuver for the IVC, dissection of the left renal vein, and mobilization of the hemilateral kidney have been described to obtain optimal cytoreduction without an increase in bleeding 1. Conversely, para-aortic lymphadenectomy could eliminate the injurious symptoms related to para-aortic LN metastasis, which includes ureteral obstruction and back pain.
Although open surgery has been initially indicated, minimally invasive lymphadenectomy for ILNRs from gynecological cancers has more recently exhibited promising surgical outcomes. In 2008, Moreno et al. 16 described eight cases of extraperitoneal laparoscopic para-aortic lymphadenectomy for suspected LN recurrence with favorable surgical outcomes. They concluded that laparoscopic para-aortic lymphadenectomy should be an option for retroperitoneal ILNR 16. In 2010, Franco-Camps et al. 17 reported 15 cases of extraperitoneal laparoscopic para-aortic lymphadenectomy with complete debulking surgery of suspicious lymphadenopathy. Post-operatively, they noted a very low complication rate, low blood loss, and reduced duration of hospitalization, allowing for quicker patient recovery 17. In 2011, Hong et al. 18 assessed the viability and efficacy of laparoscopic lymphadenectomy in six patients with ILNR who underwent primary surgery for gynecological malignancies. In their series, all procedures were correctly performed without complications, and they concluded that laparoscopic lymphadenectomy for ILNR was feasible 18. Currently, the largest series of ILNR treated by a minimally invasive approach was reported by Gallotta et al., in 2018 19. In their study, they retrospectively reviewed the data of 40 patients with ILNR due to gynecological cancers who underwent minimally invasive lymphadenectomy (31 laparoscopic, 9 robotic) over a period of 4 years (2013–2017). The ILNR occurred most frequently in the aortic region (47.5%). No case was converted to open surgery, and the median operative time, estimated blood loss, and post-operative hospitalization were 220 min, 80 mL, and 2 days, respectively. They reported two (5.0%) intra-operative and four (10.0%) postoperative cases of complications (two were grade 3). In all cases, they concluded that minimally invasive resection of LN recurrence resulted in the complete elimination of the disease with adequate surgical results, similar to those obtained with an open approach. However, none of these studies have determined the optimal upper limit of the tumor size that can be treated via a laparoscopic approach. Moreover, to date, there are no proven laparoscopic procedures that can be used for performing lymphadenectomy for ILNRs. At our institution, we believe that the wise use of different devices is the key to performing surgery with a low incidence of complications. Despite this, ILNR lymphadenectomy can lead to high surgical stress, nerve injury, protracted ileus, lymphorrhea, and chylous ascites. As such, this surgical approach should be indicated after acknowledging the potential therapeutic advantages as well as the side effects.
In our study, we reported 27 cases of laparoscopic lymphadenectomy for ILNR; among these, three had remarkably large LNs. Notably, we successfully resected one metastatic node measuring 5.5 × 5.5 × 2.5 cm at the level of the IVC and another one at the level of the aorta with adhesions to the mesenteric artery (8 × 8 × 6 cm). Resection of such large masses in a minimally invasive way was made possible through the expert use of various devices, such as an ultrasonic dissector (Harmonic Scalpel, Ultracision, Ethicon Endosurgery Inc., Cincinnati OH), LigaSure Maryland Jaw Laparoscopic Sealer/Divider (Covidien, Boulder, CO), LigaSure Blunt Tip Laparoscopic Sealer/Divider (Covidien, Boulder, CO), BiClamp® LAP and BiClamp® LAP Maryland Forceps (Erbe, Germany), the 5-mm Endo Peanut Blunt Dissector (Covidien, Boulder, CO), 10-mm Endopath blunt Cherry Dissector (Ethicon, Hamburg, Germany), and gauzes, as shown in the supplementary videos.
Based on the findings of the cited studies and our results, laparoscopic lymphadenectomy for ILNR is feasible, safe, and provides optimal surgical outcomes. Moreover, it facilitates obtaining a precise intraoperative diagnosis with histopathological confirmation of the relapse, and through its minimally invasive nature, a quicker recovery and early initiation of subsequent systemic chemotherapy. This point may be crucial in allowing optimal and timely integration of a multimodal approach that would be useful in improving the oncological outcome in patients with recurrent gynecological cancers. Additionally, the laparoscopic approach could be advantageous in providing a maximum focus on supporting the physical and psychological well-being of the patient, which would also contribute to preserving the integrity of the patient’s body image.
Our results showed that a minimally invasive approach is feasible and safe even for large masses up to 8 cm, as described in detail in the emblematic case reports; thus, contributing useful information for the definition of the upper limit of nodal size for laparoscopic surgery. In such recurrences, the treatment plan must be selected based on several parameters, comprising the site of recurrence, level of infiltration, previous treatments, chemo- and/or radio-sensitivity, and patients’ features. Another key factor is the surgeon’s skills, as these procedures are technically difficult. Therefore, the indications for a minimally invasive approach must be based both on the patient’s characteristics and on the expertise of the surgical team.
We believe that surgeons who undertake the laparoscopic surgical approach for ILNR may benefit from the following learning points: 1) adequate and rigorous training of surgeons who intend to undertake minimally invasive surgical approaches; 2) use of the most appropriate devices for coagulation and tissue resection, including the simultaneous use of the biclamp coagulation devices; 3) use of appropriate forceps for the vascular structures; 4) the use of the most appropriate devices for hemostasis and dissection; 5) skilled and expert surgeon to deal with potential vascular damage or injury to adjacent organs (i.e. bowel, ureter, and nerves) or alternatively, the presence of a multidisciplinary team.
A strength of our study is its prospective design, compared to most data in the literature that are derived retrospectively. Moreover, our report demonstrated the use of minimally invasive surgery for safe and successful resection of very large nodal masses reaching dimensions that have rarely been approached laparoscopically.
A limitation of our study was that the decision to perform the minimally invasive surgery was based on the expertise of a single surgeon, which could have had an impact on the positive surgical outcomes. Another limitation may be that the follow-up from secondary surgery for the most recent cases may not have been long enough to obtain more data for conclusive results regarding the long-term oncological outcomes. Moreover, our sample size was limited; therefore, considering that most previous studies have been observational, single-center, and with small sample sizes, further, larger, multicentric, prospective, and comparative studies are warranted to provide more evidence to support our results and establish the ideal approach for these patients.
5. Conclusions
Minimally invasive surgery for ILNR in gynecological malignancies may be an option technically feasible, safe, and effective in terms of oncological outcomes, even for large tumors, although additional research is required. It can facilitate the early initiation of subsequent appropriate systemic anticancer treatments and allow the preservation of the patient’s physical integrity and well-being.
Funding
This research was funded by Fondazione di Sardegna, grant number 2021. 1517. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical Approval
According to the Italian Regulatory Agency for observational trials not involving drugs, the protocol was notified and approved by the Institutional Review Board of Businco Hospital on 15 November 2021.
Author contribution
Conceptualization, A.M., E.S., C.M.; methodology, E.S., C.M., F.L., S.O., M.S., P.G.C., and A.M.; validation, E.S., C.M., M.S., P.G.C., and A.M.; formal analysis, E.S., C.M., F.L., S.O., M.S., P.G.C., and A.M.; investigation, E.S., C.M., F.L., S.O., M.S., P.G.C., and A.M.; resources, C.M., and A.M.; data curation, E.S., C.M., F.L., S.O., M.S., and A.M.; writing—original draft preparation, E.S., C.M., M.S., and A.M.; writing—review and editing, E.S., C.M., F.L., S.O., M.S., P.G.C., and A.M.; supervision, A.M.; project administration, A.M. and C.M.; funding acquisition, A.M. and C.M. All authors have read, revised and approved the final submitted version of the manuscript.
Guarantor
Antonio Macciò, Clelia Madeddu.
Data availability statement
Prospective anonymized data were collected on all consecutive cases and held locally in a secure database at the Department of Gynecologic Oncology, A. Businco Hospital, ARNAS G. Brotzu, Cagliari, and are available on request from the corresponding author.
Declaration of competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
The work was supported by “Associazione Sarda per la Ricerca in Ginecologia Oncologica-ONLUS”.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Video 1: Secondary cytoreductive surgery in a case of ILNR from high-grade serous ovarian cancerSupplementary Video 2: Secondary cytoreductive surgery in a case of ILNR form uterine sarcoma.
References
[1] K. Kato, K. Omatsu, N. Takeshima Secondary debulking surgery in ovarian cancer patients with isolated nodal recurrence located in the region above and behind the renal vein Gynecol. Oncol., 130 (2013), pp. 226-228, 10.1016/j.ygyno.2013. 03.025
[2] A. Ferrero, A. Ditto, G. Giorda, A. Gadducci, S. Greggi, A. Daniele, L. Fuso, E. Panuccio, C. Scaffa, F. Raspagliesi, P. Sismondi, N. Biglia Secondary cytoreductive surgery for isolated lymph node recurrence of epithelial ovarian cancer: a multicenter study Eur. J. Surg. Oncol., 40 (2014), pp. 891-898, 10.1016/j.ejso. 2013.11.02 6
[3] P. Blanchard, A. Plantade, C. Pagès, P. Afchain, C. Louvet, C. Tournigand, A. de Gramont Isolated lymph node relapse of epithelial ovarian carcinoma: outcomes and prognostic factors Gynecol. Oncol., 104 (2007), pp. 41-45, 10.1016/j.ygyno. 2006.06.039
[4] L.G. Sapienza, M.J.L. Gomes, V.F. Calsavara, M.M. Leitao, G. Baiocchi Does para-aortic irradiation reduce the risk of distant metastasis in advanced cervical cancer? A systematic review and meta-analysis of randomized clinical trials Gynecol. Oncol., 144 (2017), pp. 312-317, 10.1016/j.ygyno.2016.11.044
[5] N.R. Abu-Rustum, D.S. Chi, M. Leitao, E.A. Oke, M.L. Hensley, K.M. Alektiar, R.R. Barakat What is the incidence of isolated paraaortic nodal recurrence in grade 1 endometrial carcinoma? Gynecol. Oncol., 111 (2008), pp. 46-48, 10.1016/j.ygyno.2008.06.010
[6] Y. Niibe, M. Kenjo, T. Kazumoto, K. Michimoto, M. Takayama, C. Yamauchi, M. Kataoka, K. Suzuki, N. Ii, T. Uno, T. Takanaka, K. Higuchi, H. Yamazaki, S. Tokumaru, M. Oguchi, K. Hayakawa. Multi-institutional study of radiation therapy for isolated para-aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5,000. Int. J. Radiat. Oncol. Biol. Phys., 66 (2006), pp. 1366-1369, 10.1016/j.ijrobp.2006.07.1384
[7] H.H. Chou, C.C. Wang, C.H. Lai, J.H. Hong, K.K. Ng, T.C. Chang, C.J. Tseng, C.S. Tsai, J.T. Chang Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int. J. Radiat. Oncol. Biol. Phys., 51 (2001), pp. 442-448, 10.1016/s0360-3016(01)0162 8-5
[8] A.K. Singh, P.W. Grigsby, J.S. Rader, D.G. Mutch, M.A. Powell Cervix carcinoma, concurrent chemoradiotherapy, and salvage of isolated paraaortic lymph node recurrence Int. J. Radiat. Oncol. Biol. Phys., 61 (2005), pp. 450-455, 10.1016/j.ijrob p.2004.06.207
[9] A.C. Schlaerth, N.R. Abu-Rustum Role of minimally invasive surgery in gynecologic cancers Oncol., 11 (2006), pp. 895-901, 10.1634/theoncologist.11-8-895
[10] M. La Verde, G. Riemma, A. Tropea, A. Biondi, S. Cianci. Ultra-minimally invasive surgery in gynecological patients: a review of the literature Updates Surg. (2022), 10.1007/s13 304-022-01248-y
[11] R.K. Reynolds, A.P. Advincula Robot-assisted laparoscopic hysterectomy: technique and initial experience. Am. J. Surg., 191 (2006), pp. 555-560, 10.1016/j.amjsurg. 2006.01.01 1
[12] S. Cianci, M. Arcieri, G. Vizzielli, C. Martinelli, R. Granese, M. La Verde, A. Fagotti, F. Fanfani, G. Scambia, A. Ercoli Robotic pelvic exenteration for gynecologic malignancies, anatomic landmarks, and surgical steps: a systematic review. Front. Surg., 8 (2021), Article 790152,10.3389/ fsurg.2021.790152
[13] P.J. Coronado, M.A. Herraiz, J.F. Magrina, M. Fasero, J.A. Vidart. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer Eur. J. Obstet. Gynecol. Reprod. Biol., 165 (2012), pp. 289-294, 10.1016/j.ejogrb. 2012.07.006
[14] A. Buia, F. Stockhausen, E. Hanisch. Laparoscopic surgery: a qualified systematic review World J. Methodol., 5 (2015), pp. 238-254, 10.5662/wjm. v5.i4.238
[15] P.T. Ramirez Laparoscopic surgery for isolated nodal recurrence: appropriate for all patients or only a select few? J. Minim. Invasive Gynecol., 19 (2012), pp. 146-147, 10.1016/j.jmig.2012. 01.006
[16] A. Gil-Moreno, S. Franco-Camps, B. Díaz-Feijoo, A. Pérez-Benavente, J.M. Martínez-Palones, J.M. Del Campo, M. Parera, R. Verges, J. Castellví, J. Xercavins. The usefulness of extraperitoneal laparoscopic paraaortic lymphadenectomy for lymph node recurrence in gynecologic malignancy. Acta Obstet. Gynecol. Scand., 87 (2008), pp. 723-730, 10.1080/00016340802136343
[17] S. Franco-Camps, S. Cabrera, A. Pérez-Benavente, B. Díaz-Feijoo, M. Bradbury, J. Xercavins, A. Gil-Moreno Extraperitoneal laparoscopic approach for diagnosis and treatment of aortic lymph node recurrence in gynecologic malignancy J. Minim. Invasive Gynecol., 17 (2010), pp. 570-575, 10.1016/ j.jmig.2010.03.020
[18] J.H. Hong, J.S. Choi, J.H. Lee, J.W. Bae, J.M. Eom, J.T. Kim, S. Oh. Laparoscopic lymphadenectomy for isolated lymph node recurrence in gynecologic malignancies. J. Minim. Invasive Gynecol., 19 (2012), pp. 188-195,10.1016/j.jmig.2011.10. 013
[19] V. Gallotta, M.T. Giudice, C. Conte, A.V. Sarandeses, M. D’Indinosante, A. Federico, L. Tortorella, M.V. Carbone, S. Gueli Alletti, G. Vizzielli, B. Costantini, G. Scambia, G. Ferrandina Minimally invasive salvage lymphadenectomy in gynecological cancer patients: a single institution series. Eur. J. Surg. Oncol., 44 (2018), pp. 1568-1572, 10.1016/j.ejso.2018. 08.006
[20] S. Kusunoki, K.G. Huang, A. Magno, C.L. Lee. The laparoscopic technique of para-aortic lymph node dissection: a comparison of the different approaches to trans- versus extraperitoneal para-aortic lymphadenectomy. Gynecol. Minim. Invasive Ther., 6 (2017), pp. 51-57, 10.1016/j.gmit.2016.01.003
[21] R.A. Agha, C. Sohrabi, G. Mathew, T. Franchi, A. Kerwan, N. O’Neill. PROCESS Group, the PROCESS 2020 guideline: updating consensus preferred reporting of case series in surgery (PROCESS) guidelines. Int. J. Surg., 84 (2020), pp. 231-235, 10.1016/j.ijsu.2020.11.005
[22] D. Dindo, N. Demartines, P.A. Clavien. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg., 240 (2004), pp. 205-213, 10.1097/01.sla.00001 33083.54934.ae
[23] A. Macciò, G. Chiappe, P. Kotsonis, F. Lavra, R. Nieddu, P. Onnis, E. Sanna, V. Mais, C. Madeddu. The utility of fibrinogen level as a predictor of complications after laparoscopic gynecologic surgery: a prospective observational study. Gynecol. Surg., 16 (2019), p. 11, 10.1186/s10397-019-1064-x
[24] F. Legge, M. Petrillo, V. Adamo, S. Pisconti, G. Scambia, G. Ferrandina. Epithelial ovarian cancer relapsing as isolated lymph node disease: natural history and clinical outcome. BMC Cancer, 8 (2008), p. 367, 10.1186/ 1471-2407-8-367
[25] Y. Niibe, T. Kazumoto, T. Toita, H. Yamazaki, K. Higuchi, N. Ii, K. Suzuki, T. Uno, S. Tokumaru, M. Takayama, K. Sekiguchi, Y. Matsumoto, K. Michimoto, M. Oguchi, K. Hayakawa. Frequency and characteristics of isolated para-aortic lymph node recurrence in patients with uterine cervical carcinoma in Japan: a multi-institutional study. Gynecol. Oncol., 103 (2006), pp. 435-438, 10.1016/ j.ygyno.2006.03.034
[26]T.Al Al Rawahi, A.D. Lopes, R.E. Bristow, A. Bryant, A. Elattar, S. Chattopadhyay, K. Galaal. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. (2) (2013), Article Cd008 765, 10.1002/14651858.Cd008765. pub3
[27] S.M. Eisenkop, N.M. Spirtos. The clinical significance of occult macroscopically positive retroperitoneal nodes in patients with epithelial ovarian cancer. Gynecol. Oncol., 82 (2001), pp. 143-149, 10.1006/gyno.2001. 6232
[28]A. Gadducci, S. Cosio, P. Zola, B. Sostegni, A.M. Ferrero, G. Teti, R. Cristofani, E. Sartori. The clinical outcome of epithelial ovarian cancer patients with apparently isolated lymph node recurrence: a multicenter retrospective Italian study. Gynecol. Oncol., 116 (2010), pp. 358-363, 10.1016/j.ygyno.2009.11.008
[29] S. Fotiou, T. Aliki, Z. Petros, S. Ioanna, V. Konstantinos, M. Vasiliki, C. George. Secondary cytoreductive surgery in patients presenting with isolated nodal recurrence of epithelial ovarian cancer. Gynecol. Oncol., 114 (2009), pp. 178-182, 10.1016/j.ygy no.2009.04.025
[30] C. Uzan, P. Morice, A. Rey, P. Pautier, S. Camatte, C. Lhommé, C. Haie-Meder, P. Duvillard, D. Castaigne. Outcomes after combined therapy including surgical resection in patients with epithelial ovarian cancer recurrence(s) exclusively in lymph nodes. Ann. Surg Oncol., 11 (2004), pp. 658-664, 10.1245/ASO. 2004.11.023
[31] T. Kato, K.H. Seol, J.S. Youn, D.G. Hong. Salvage para-aortic lymphadenectomy in recurrent cervical cancer after visualization with 3-dimensional computed tomography angiography. Obstet. Gynecol. Sci., 61 (2018), pp. 626-630, 10.5468/ogs.2018.61.5. 626
[32] P.B. Panici, M. Calcagno, F. Plotti, C. Arrivi, V. Di Donato, R. Montera, R. Angioli. Aortic lymphadenectomy in cervical cancer: anatomy, classification, and technique. Gynecol. Oncol., 107 (Supplement 1) (2007), 10.1016/j.ygyno.2007.07.029 S30–S32
[33] H. Kubota, K. Tsujino, N.S. Sulaiman, S. Sekii, Y. Matsumoto, Y. Ota, T. Soejima, S. Yamaguchi, R. Sasaki. Comparison of salvage therapies for isolated para-aortic lymph node recurrence in patients with uterine cervical cancer after definitive treatment. Radiat. Oncol., 14 (2019), p. 236, 10.1186/s13014-019-1442-6.
[34] J.N. Barlin, I. Puri, R.E. Bristow Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol. Oncol., 118 (2010), pp. 14-18, 10.1016/j.ygyno. 2010.04.005
Credits: Sanna E, Madeddu C, Lavra F, Oppi S, Scartozzi M, Giorgio Calò P, Macciò A. Laparoscopic management of isolated nodal recurrence in gynecological malignancies is safe and feasible even for large metastatic nodes up to 8 cm: A prospective case series. Int J Surg. 2022 Aug;104:106744. doi: 10.1016/j.ijsu. 2022.106744. Epub 2022 Jul 1. PMID: 35787955.