Fleur L Meulmeester, MSca ∙ Samuel Mailhot-Larouche, MDb ∙ Carlos Celis-Preciado, MDb ∙ Samuel Lemaire-Paquette, MScc ∙ Sanjay Ramakrishnan, PhDd ∙ Prof Michael E Wechsler, MDe ∙ Prof Guy Brusselle, MDf ∙ Prof Jonathan Corren, MDg ∙ Jo Hardy, PhDh ∙ Sarah E Diver, PhDi ∙ Prof Christopher E Brightling, FMedScii ∙ Prof Mario Castro, MDj ∙ Prof Nicola A Hanania, MDk ∙ Prof David J Jackson, PhDl ∙ Prof Neil Martin, MDi,m ∙ Annette Laugerud, MDm ∙ Emilio Santoro, PhDn ∙ Chris Compton, MDn ∙ Megan E Hardin, MDo ∙ Cecile T J Holweg, PhDp ∙ Allu Subhashini, MBBSq ∙ Timothy S C Hinks, PhDr ∙ Prof Richard W Beasley, DScs ∙ Jacob K Sont, PhDa ∙ Prof Ewout W Steyerberg, PhDa,t ∙ Prof Ian D Pavord, FMedScir ∙ Simon Couillard, MDa,b,r
Summary
Background
Clinical risk factors for severe asthma attacks have been identified, but their incremental prognostic values are unclear. Additionally, the incremental contribution of type 2 inflammation, a common and treatable process, remains undetermined. We aimed to quantify the prognostic value of baseline characteristics and type 2 inflammatory biomarkers, specifically blood eosinophil count and fractional exhaled nitric oxide (FeNO), to predict asthma attacks.
Methods
In this systematic review and meta-analysis of randomised controlled trials (RCTs), Oxford Asthma Attack Risk Scale 2 (ORACLE2), we searched MEDLINE from Jan 1, 1993, to April 1, 2021, for trials investigating fixed treatment regimen effects on asthma attack rates for at least 6 months with baseline blood eosinophil count and FeNO. Eligible participants were aged 12 years or older with asthma (any severity) who had been randomly assigned to the control group of an RCT. Relevant trials were manually retrieved and reviewed by two independent reviewers (SC and IDP). Disagreements were discussed with five reviewers. Individual patient data (IPD) for meta-analysis were requested from the study authors. We investigated the rate of severe asthma attacks (≥3 days of systemic corticosteroids) for at least 6 months and the prognostic effects of baseline blood eosinophil count and FeNO in control group participants. Rate ratios (RRs) with 95% CIs were derived for annualised asthma attack rates from negative binomial models adjusted for key variables, including blood eosinophil count and FeNO, and interactions between these type 2 inflammatory biomarkers were explored. Certainty of evidence was assessed using GRADE. The heterogeneity of the included studies and potential for ecological bias were quantified by the concordance statistic (C-statistic). This study was registered with PROSPERO, CRD42021245337.
Findings
We identified 976 potentially eligible studies. After automated screening, we manually reviewed 219 full-text articles. Of these, 19 publications comprising 23 RCTs were eligible. 6513 participants (4140 [64%] female; 2370 [36%] male; three missing) spanning 22 RCTs were included for data analysis. 5972 (92%) of 6513 patients had moderate-to-severe asthma. 4615 asthma attacks occurred during 5482 person-years of follow-up (annualised rate 0·84 per person-year). Higher blood eosinophil count or FeNO was linked to higher asthma attack risk (per 10-fold increase, RR 1·48 [95% CI 1·30–1·68] for blood eosinophil count and 1·44 [1·26–1·65] for FeNO; high-certainty evidence). Other prognostic factors were attack history (yes vs no, RR 1·94 [1·61–2·32]); disease severity (severe vs moderate, RR 1·57 [1·22–2·03]); FEV1 percentage predicted (FEV1%; per 10% decrease, RR 1·11 [1·08–1·15]); and 5-item Asthma Control Questionnaire score (ACQ-5; per 0·5 increase, RR 1·10 [1·07–1·13]). High blood eosinophil count and FeNO combined were associated with a greater risk than either prognostic factor separately. Bronchodilator reversibility was associated with a lower risk of severe asthma attacks (per 10% increase, RR 0·93 [0·90–0·96]), with the reduction observed primarily between 0% and 25%. Regarding heterogeneity of the included studies, the C-statistic ranged from 0·58 to 0·95, indicating major differences in patient and disease characteristics between studies. In the univariable meta-analysis per trial, we found substantial heterogeneity in associations between studies, with I2 statistics ranging from 0·56 to 0·97.
Interpretation
Blood eosinophil count, FeNO, asthma attack history, disease severity, low lung function (low FEV1%), and symptoms (ACQ-5 score) are key predictors of asthma attacks. Conversely, we found that moderate bronchodilator reversibility was associated with reduced risk. These findings from high-quality multinational RCTs support the incorporation of blood eosinophils and FeNO into clinical risk stratification for targeted risk reduction. More individualised clinical decision-making models should be explored.
Funding
National Institute of Health and Care Research Oxford Biomedical Research Centre; Association pulmonaire du Québec; Fonds de recherche du Québec—Santé; Québec Air-Intersectorialité-Respiratoire-Son network; Stichting Astma Bestrijding; Leiden University Fund; and Academy of Medical Sciences
Introduction
Asthma is a chronic disease affecting 400 million people worldwide and 10% of high-income country populations.1 Due to morbidity, mortality, and health-care costs, asthma attacks are key outcomes that need to be predicted and prevented.1,2
Evidence supports risk assessment and treatment titration based on a history of a previous asthma attack and a list of clinical risk factors.1 This framework is centred on symptoms (eg, night-time awakenings, activity limitations, and frequent reliever requirement) and evidence of damage (eg, lung function decline and previous attacks), which are identifiable only after they have happened. The limitations of this approach are that many of these prognostic factors, such as history of a previous asthma attack or sex assigned at birth, do not inform on modifiable pathways. Other factors, such as lung function and symptoms, are modifiable independently of an effect on asthma attacks. For example, long-acting bronchodilator monotherapy improves airflow and symptoms without reducing asthma attack risk.3 Furthermore, lung function must decline to gain prognostic value, implying that the airways must be damaged to prompt more aggressive management. Identifying risk factors that inform a causal and treatable biological pathway that underpins the adverse outcome of interest might be more useful than symptom management to improve outcomes.4
The past decade of research has revealed that type 2 inflammation is prevalent, measurable, treatable, and, in many cases, a cause of asthma attacks.5–8 Type 2 inflammation is identifiable in the clinic by the use of two independent, complementary, and accessible biomarkers: the peripheral blood eosinophil count and fractional exhaled nitric oxide (FeNO).5,9 Importantly, inflammation and the risk of attacks identified by these biomarkers are reduced with appropriate treatment,7 be it low-dose inhaled corticosteroids in mild asthma,10,11 a higher dose of inhaled corticosteroids in moderate asthma,12,13 or biological agents targeting type 2 inflammatory pathways in moderate and severe asthma.14–17 Blood eosinophils and FeNO have thus emerged as so-called treatable traits, crucial for redefining airway diseases.5
Research in context
Evidence before this study
Although risk factors for asthma attacks have been identified, there is limited understanding of the quantitative risk associated with these characteristics. We reviewed the 2023 findings of an annual, independent, international expert literature review that identified risk factors for asthma attacks. The list of 20 risk factors was based on 35 publications, none of which informed the multivariable prognostic relations across clinical profiles. We also reviewed 12 previously published clinical prediction models for asthma attacks and found none that included both blood eosinophil count and fractional exhaled nitric oxide (FeNO) as prognostic variables. Finally, we searched PubMed from Jan 1, 1993, to Feb 23, 2024, for clinical trials, systematic reviews, and meta-analyses assessing risk factors for asthma attacks. We used the search terms “asthma”, “exacerbation”, “risk”, and “factors” with no language or time restrictions. This search yielded 192 results, including ten papers with multivariable analyses to predict asthma attacks. Only one publication included blood eosinophils and FeNO in their multivariable prediction model of asthma attacks. However, this analysis was a real-world randomised trial in adolescents in which background treatment fluctuated.
Added value of this study
We collaborated with academic, public, and pharmaceutical data providers to access high-quality individual patient data (IPD) from the control groups of 22 randomised controlled trials of asthma, for a total of 4615 attacks over 5482 person-years of follow-up. This first broad consortium in airway disease enabled us to perform a large IPD meta-analysis, providing precise estimates of the prognostic value of type 2 inflammatory biomarkers and other characteristics while background therapy was controlled. Our multivariable analyses show that raised blood eosinophil count and FeNO are important, multiplicative, and prevalent predictors of asthma attacks, with synergistic prognostic effects (high-certainty evidence). The prognostic effects of blood eosinophil count and FeNO were incremental to other key risk factors, which our model adjusted for: asthma attack history, disease severity, lower lung function, and symptoms (high-certainty evidence). After adjusting for the presence of type 2 inflammation, we showed that moderate bronchodilator reversibility—traditionally considered the defining characteristic and diagnostic gold standard for asthma—was associated with reduced asthma attack risk (moderate-certainty evidence). These findings imply that clinical risk stratification in asthma should incorporate inflammatory pheno-typing using blood eosinophil count and FeNO.
Implications of all the available evidence
This work quantifies the value of measurable inflammatory and clinical prognostic factors to identify patients at the highest risk of asthma attacks. Unlike a predictor such as previous history of asthma attacks, tools such as the type 2 inflammatory biomarkers anticipate risk and provide opportunities to intervene preventively. In our large IPD meta-analysis, elevations in blood eosinophil count and FeNO were associated with an excess risk of asthma attacks. Considering the mechanistic, prognostic (ie, predicting adverse outcomes), and theragnostic (ie, predicting treatment responsiveness) values of type 2 inflammatory biomarkers, we speculate that individualised clinical decision making in asthma would be improved by a framework that includes these modifiable prognostic factors in clinical prediction models.
We previously proposed a proof-of-concept biomarker-stratified asthma attack scale, suggesting a novel framework for clinical decision making.6,7 This conceptual prototype did not have a detailed and statistically robust assessment of multivariable prognostic relations and interactions. Importantly, previous studies have not yet shown the synergistic value of biomarkers in predicting asthma attacks, probably due to the correlation between the biomarkers and sample size constraints in previous analyses. In this individual patient data (IPD) meta-analysis, we aimed to quantify the prognostic value of baseline characteristics and type 2 inflammatory biomarkers, specifically blood eosinophil count and FeNO, to predict asthma attacks.
Methods
Search strategy and selection criteria
The study protocol was published and followed without deviation for this systematic review and meta-analysis.18 We followed the PRISMA-IPD and REMARK checklists. 19,20 Eligible studies were randomised controlled trials (RCTs) published from Jan 1, 1993, to April 1, 2021, investigating fixed treatment regimen effects on asthma attack rates for 6 months or more, with baseline blood eosinophil count and FeNO. We searched MEDLINE using previously described terms (appendix p 4),18 with language restricted to English and French. Relevant trials were manually retrieved. Two reviewers (SC and IDP) independently reviewed retained publications to select trials for inclusion. Disagreements were discussed (SC, IDP, EWS, JKS, and FLM), with reasons for exclusion recorded. There were no specific trial exclusion criteria. Trial inclusion was unblinded. Authors of retained studies and trial sponsors were contacted and invited to contribute IPD and join the Oxford Asthma Attack Risk Scale 2 (ORACLE2) consortium. We contacted and received responses from all authors of the included trials. Manual reference searching was performed for completed clinical trials that were in press at the time of the systematic review, which were identified through press releases.
We included IPD from patients aged 12 years or older diagnosed with asthma of any severity according to objective criteria who were randomly assigned to the control group of an RCT. The control group was the intervention corresponding to the lowest anti-inflammatory therapy intensity after randomisation (ie, randomly assigned to no inhaled corticosteroids, lowest-dose inhaled corticosteroids, or inhaled corticosteroids plus placebo). We excluded patients allocated to receive inhaled corticosteroids in the control group if they were not on inhaled corticosteroids before randomisation.21 We also excluded patients missing data for both the baseline blood eosinophil count and FeNO, asthma severity (Global Initiative for Asthma [GINA] treatment steps: 1 being very mild to 5 being severe asthma), follow-up duration, or number of severe asthma attacks during follow-up, or who did not consent to third-party data sharing.
Each study was analysed with the Cochrane Collaboration tool for assessing the risk of bias in randomised trials.22 These judgments were made independently by two authors (CC-P and SC) based on the criteria for judging the risk of bias across five domains. Disagreements were resolved by discussion or consortium arbitration.
Data analysis
We requested anonymised IPD for 42 covariates (appendix pp 46–50). These data included demographics, GINA treatment step, baseline spirometry, severe asthma attack history, control group intervention, biomarkers, other GINA-defined risk factors at baseline,1 duration of follow-up under control therapy, and the outcome of interest (ie, the number of severe asthma attacks during follow-up). Severe asthma attacks were defined as acute asthma episodes requiring at least 3 days of systemic corticosteroids. Data were shared freely. The external data extraction programming code was requested and made available on GitHub whenever possible.
Extracted data were securely transferred to a digital storage solution provided by the University of Oxford. Under the terms of the data sharing agreements, access to the complete dataset was available to the primary authors and statisticians on the study protocol (FLM, EWS, IDP, and SC).
IPD were combined for the initial data analysis (IDA).23 The IDA was conducted by two authors (FLM and SC) and comprised cleaning and screening of the extracted data, (graphical) description of the data distribution and missingness, and assessment of missingness. Detailed IDA steps are reported in the appendix (pp 4–7). Throughout the IDA process, discrepancies were resolved by consensus discussion, in direct collaboration with data providers. There was no duplicate data. In addition, R coding was supported by two independent statisticians. The heterogeneity of the included studies and potential for ecological bias were quantified by a membership concordance statistic (C-statistic). A high C-statistic reflects substantial differences between baseline characteristics of the patients in distinct studies. Outcome-specific heterogeneity across trials was quantified by I2 in univariable forest plots. All statistical analyses were performed with R version 4.4.1.3 The R code for data extraction and analysis is provided on GitHub.
The assessment of missingness and imputation methods is detailed in the appendix (pp 5–7). Briefly, the 42 covariates revealed patterns of systematically missing data within some trials (appendix pp 10–13). Missing values were estimated with multiple imputation by chained equations and the mice package in R, with results combined with Rubin’s rules.24 There were small discrepancies between some variable definitions across trials (appendix, p 5). In some instances, more than 50% of observations were systematically missing in a variable, but key variables used in the analyses were not systematically missing (appendix, p 13). Candidate predictors were sorted by standardisation and completeness. For example, blood eosinophil count, FeNO, IgE, age, BMI, sex, 5-item Asthma Control Questionnaire score (ACQ-5), spirometry, the number of severe asthma attacks in the previous 12 months, and treatment step had the most standard and complete reporting across trials (appendix p 73).
To assess the prognostic relationships between baseline characteristics and the risk of asthma attacks, we derived univariable and multivariable regression coefficients from negative binomial models for the annualised asthma attack rate using the R package MASS. To account for clustering of observations within studies, we specified enrolled trial as a factor in both the univariable (model one) and the main multivariable (model two) fixed-effects models. The univariable model assessed the variable of interest while accounting for log-transformed follow-up duration as an offset variable and enrolled trial as a factor. The main multivariable model further adjusted for covariates of asthma severity (treatment step 1–5), attack history of the previous year (any attack: yes or no), mean ACQ-5 symptom score, FEV1 percentage predicted (FEV1%) prebronchodilator, log-transformed blood eosinophil count, and log-transformed FeNO (both per 10-fold increase). These covariates were selected based on the conclusions of a previous systematic review of prediction models in asthma,25 the expertise of the study statisticians and clinical experts, and the main study hypothesis that type 2 biomarkers are attractive predictors of risk that identify anti-inflammatory treatment opportunities while also being related to other prognostic factors.5–7
We quantified the incremental effects of each potential prognostic factor by clinically relevant changes in those variables (eg, typical treatment-associated changes in FeNO and blood eosinophil count, minimal clinically important differences in ACQ-5 and FEV1%). Nagelkerke R2 was calculated for the multivariable model with the R package fmsb. Further univariable and multivariable rate ratios (RRs) and 95% CIs were derived for other prognostic factors with retrievable data, sequentially adding in and then removing each factor in the main multivariable model.1 A sensitivity analysis was conducted by repeating the univariable analyses per variable of interest for each trial separately. The trial-specific RRs (95% CIs) were subsequently pooled by person-years of follow-up to account for variations in trial characteristics. The relationship between a study’s effect size and its precision was visualised for all analyses separately with funnel plots. Additionally, we performed two sensitivity analyses by excluding open-label trials and by excluding trials that we considered as having some risk of bias. We did not adjust for multiplicity since multiple prognostic factors were each considered relevant.26 A spline curve was used to explore the prespecified 18 interaction analysis between biomarkers, blood eosinophil count and FeNO with the annualised severe asthma attack rate (appendix pp 7–8). Finally, we explored the effect of FEV1 postbronchodilator reversibility on the annualised severe asthma attack rate with splines.
We assessed the certainty of evidence using GRADE27 for each prognostic factor. For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from high quality by one for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, or imprecision of effect estimates.
Ethical approval for individual studies was obtained from multiple local committees that were part of the underlying trials. We used anonymised data for secondary analysis and obtained additional ethical approval from the Oxford Tropical Research Ethics Committee. This systematic review and meta-analysis were registered with PROSPERO (CRD42021245337), and the protocol was previously published.18
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
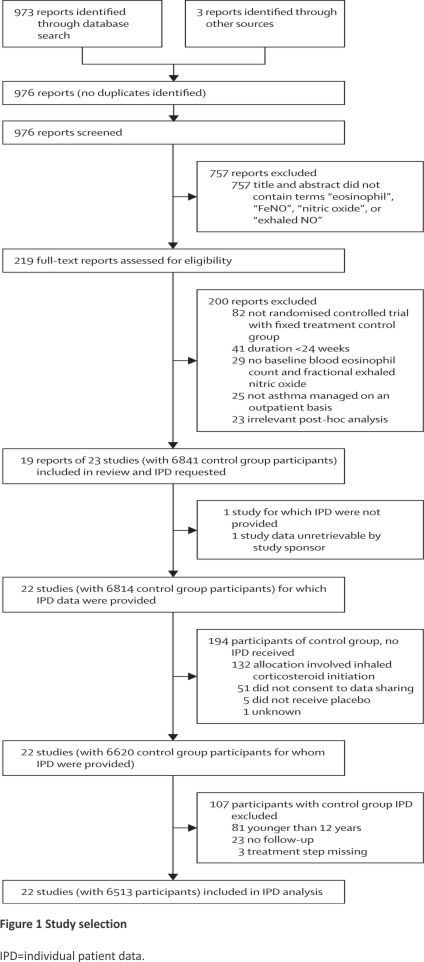
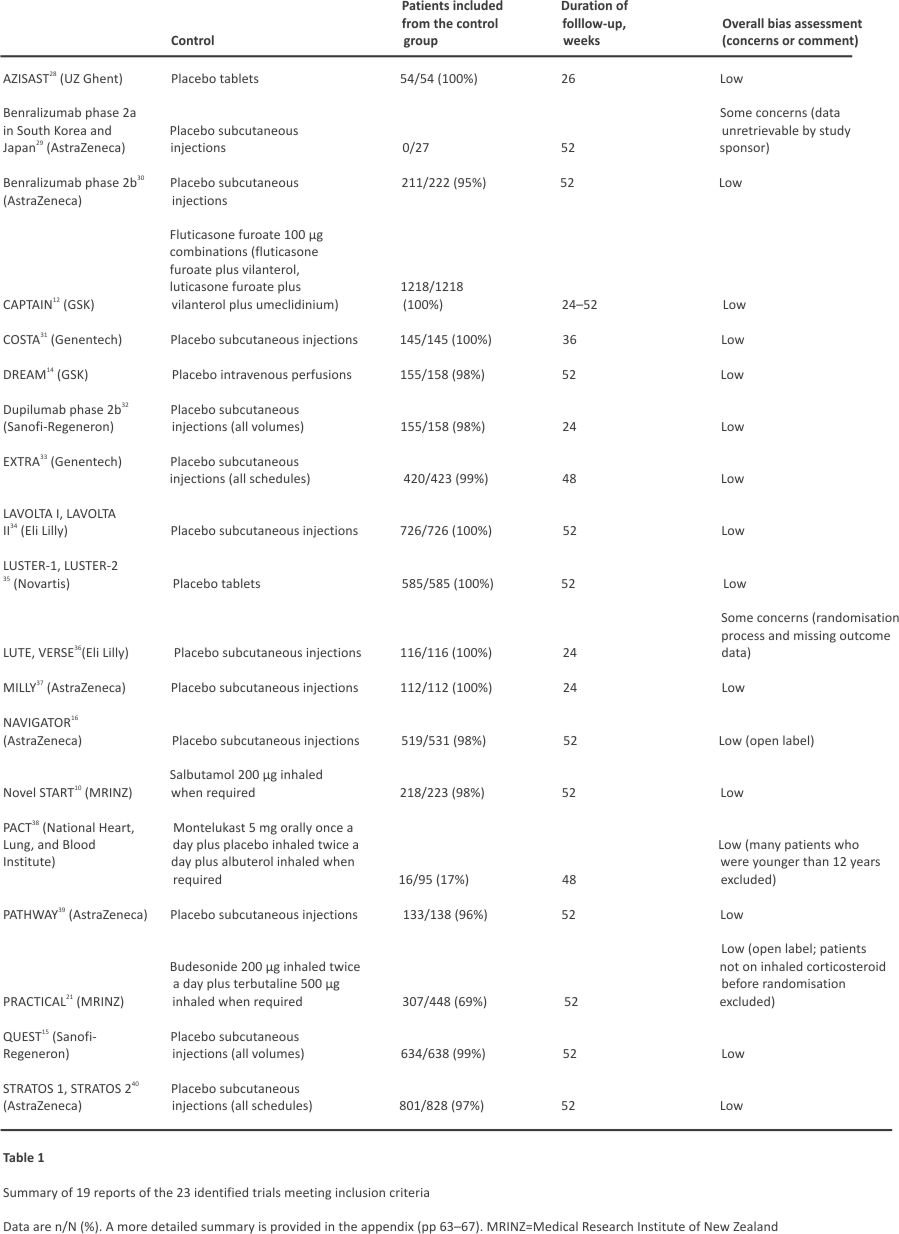
We identified 976 reports in the literature review. After automated screening of titles and abstracts, we manually reviewed 219 full-text articles (appendix pp 52–66). Of these, 19 publications comprising 23 RCTs met our eligibility criteria (Figure 1). Over 2·5 years, nine data sharing agreements were reached with three academic or public data providers and six pharmaceutical data providers to pool IPD from control groups centrally. An overview of the 23 identified RCTs is shown in Table 1, with detailed study characteristics of the analysed trials provided in the appendix (pp 74–78). Data from one small RCT were no longer retrievable.29 Of the 6841 control group patients for whom data were requested, 6513 participants spanning 22 RCTs were included for data analysis (Figure 1). All trials except two had a low risk of bias (Table 1; appendix p 14)36, and there was no relevant overlap of participants. Of the 22 analysed trials, 20 were double-blind and two were open-label RCTs.
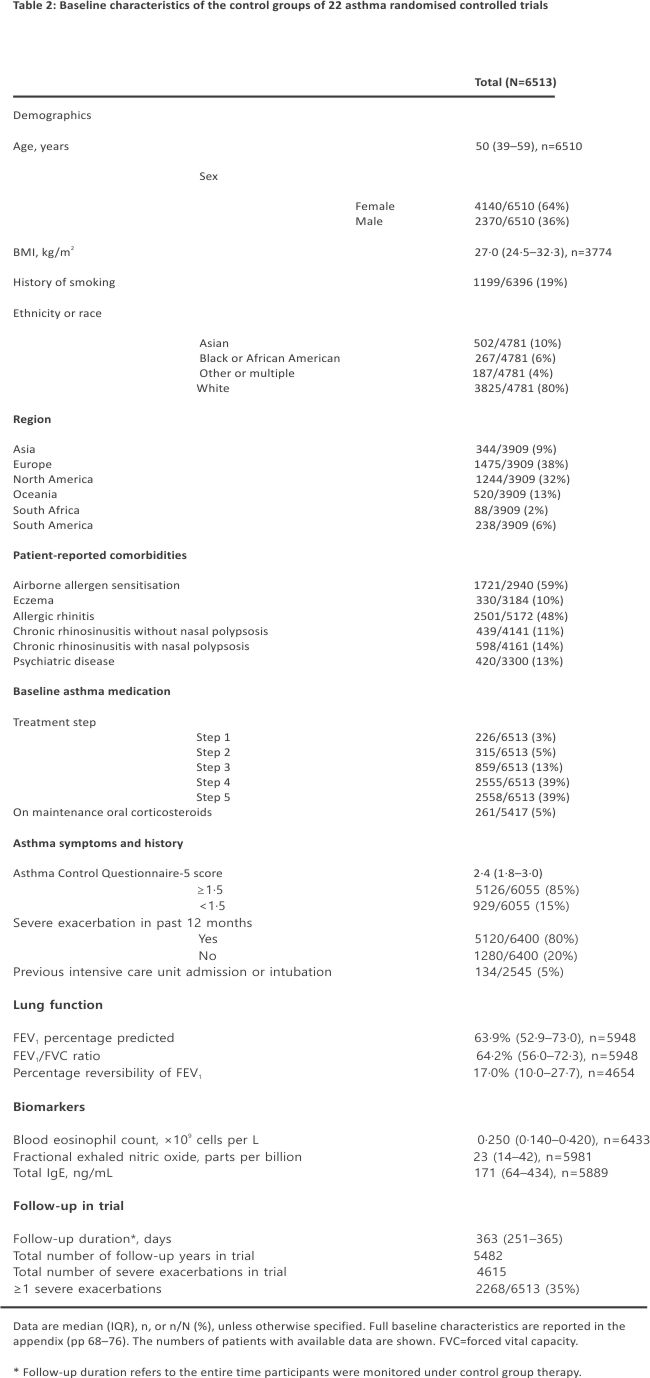
5972 (92%) of 6513 patients had moderate-to-severe asthma (treatment steps 3–5). The majority of participants had a history of severe attacks or poor asthma control, but 1280 (20%) of 6400 had not had an attack in the previous year, and 929 (15%) of 6055 had at least partly controlled symptoms (ACQ-5 <1·5). Overall, 4615 attacks over 5482 person-years of follow-up occurred (annualised rate 0·84 per person-year), providing ample power for regression analyses and prediction modelling (table 2).41 Extended variables and values disaggregated per trial are shown in the appendix (p 79–87).
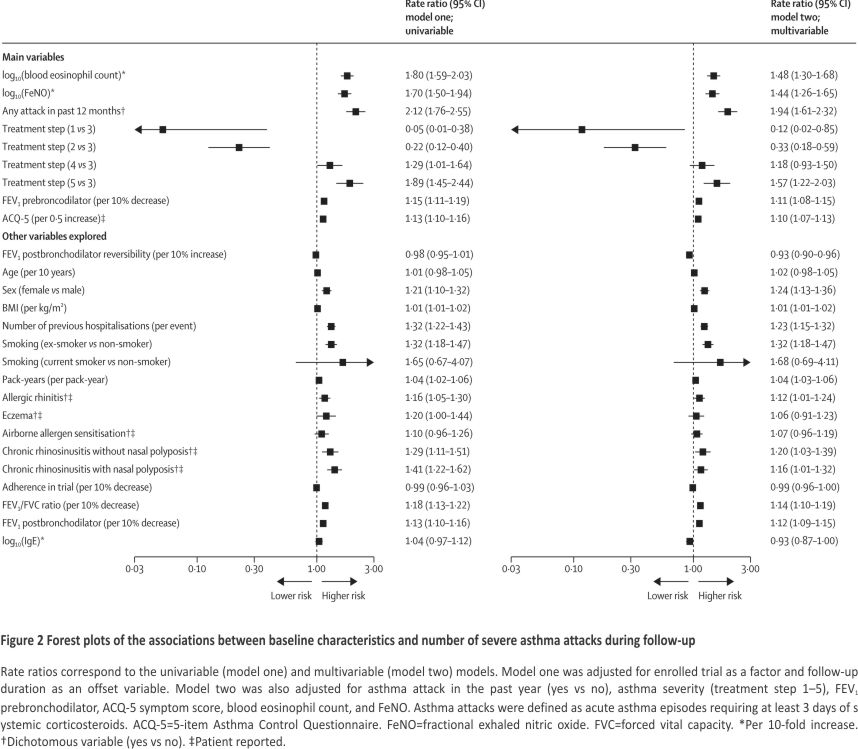
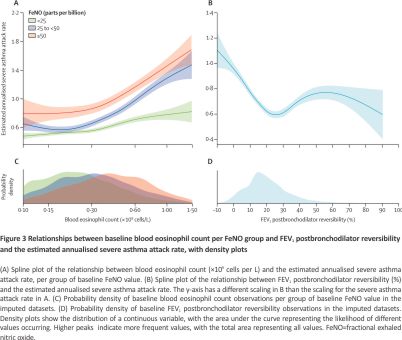
In the main multivariable regression analyses, we observed that higher blood eosinophil count or FeNO values were each associated with higher risks of asthma attacks (per 10-fold increase, RR 1·48 [95% CI 1·30–1·68] for blood eosinophil count and 1·44 [1·26–1·65] for FeNO; high-certainty evidence). Other prognostic factors (high-certainty evidence) in the main multivariable model were attack history in the previous year (yes vs no, RR 1·94 [1·61–2·32]); disease severity (severe vs moderate, RR 1·57 [1·22–2·03]); FEV1% (per 10% decrease, RR 1·11 [1·08–1·15]); and ACQ-5 (per 0·5 increase, RR 1·10 [1·07–1·13]). The model had an explained variability of 23% (adjusted R2). The results of the univariable and multivariable regression models are shown in Figure 2, with univariable outputs disaggregated per trial showing similar effect direction (appendix pp 15–35) and weighted-average effect size (appendix p 36). To assess biomarker prognostic differences further, risks at the 75th and 25th percentiles of the sample distribution were compared. A baseline blood eosinophil count of 0·42 × 109 cells per L versus 0·14 × 109 cells per L was associated with an adjusted RR of 1·16 (95% CI 1·12–1·21) and a baseline FeNO of 42 versus 14 parts per billion was associated with an adjusted RR of 1·11 (1·07–1·15; appendix p 37). RRs for 2-fold changes in blood eosinophil count and FeNO values are shown in the appendix (p 37). The absolute rates are estimated with spline curves (Figure 3 and Appendix p 38).
Among other explored variables, we found that a higher risk of a severe asthma attack was predicted by female sex (RR 1·24 [95% CI 1·13–1·36]; high certainty), higher BMI (per kg/m2, RR 1·01 [1·01–1·02]; high certainty), number of previous hospitalisations for asthma (per event, RR 1·23 [1·15–1·32]; moderate certainty, downgraded due to inconsistency in reporting), smoking status (ex-smoker vs non-smoker, RR 1·32 [1·18–1·47]; moderate certainty, downgraded due to selection bias), and greater pack-years (per pack-year, RR 1·04 [1·03–1·06]; moderate certainty, downgraded due to selection bias), allergic rhinitis (RR 1·12 [1·01–1·24]; moderate certainty, downgraded due to inconsistency in reporting), chronic rhinosinusitis with nasal polyposis (RR 1·16 [1·01–1·32]; moderate certainty, downgraded due to inconsistency in reporting), chronic rhinosinusitis without nasal polyposis (RR 1·20 [1·03–1·39]; moderate certainty, downgraded due to inconsistency in reporting), and lower FEV1/FVC ratio (per 10% decrease, RR 1·14 [1·10–1·19]; high certainty). FEV1 postbronchodilator reversibility was associated with a lower risk of severe asthma attacks (per 10% increase, RR 0·93 [0·90– 0·96]; moderate certainty due to inconsistency in effect across trials). No prognostic effect was seen for higher age (high certainty), patient reported eczema (moderate certainty, downgraded due to inconsistency in reporting), patient-reported airborne allergen sensitisation (moderate certainty, downgraded due to inconsistency in reporting), inhaled corticosteroid adherence (low certainty, down-graded due to inconsistency in reporting and over-representation of adherent patients), and serum IgE (high certainty).
When comparing the univariable and multivariable analyses, we observed that FEV1 postbronchodilator reversibility (per 10% increase) had a stronger negative prognostic value, since the RR decreased from 0·98 (95% CI 0·95–1·01) in the univariable analysis to 0·93 (0·90–0·96) in the multivariable analysis.
Sensitivity analyses excluding open-label trials (Novel START [n=218] and PRACTICAL [n=307]) and trials with some risk of bias (LUTE [n=66] and VERSE [n=50]) showed similar results (appendix pp 39–40). A post-hoc fully adjusted multivariable model (appendix p 41) showed similar prognostic effects to the main analysis.
We observed synergistic effects between blood eosinophil count and FeNO (pinteraction=0·045), as evidenced by the dissociating spline curves in Figure 3A. High type 2 inflammatory burden was prevalent in the study population (Figure 3B), an observation also found in the subset of RCTs not selecting patients according to biomarkers (appendix p 42). Synergy and high prevalence were also seen in categorical analyses, as the prespecified18 combined elevation of a blood eosinophil count of 0·30 × 109 cells per L or higher and FeNO 50 parts per billion or higher was associated with nearly double the RR compared with a blood eosinophil count of less than 0·15 × 109 cells per L and a FeNO less than 25 parts per billion (RR 1·47 [95% CI 1·30–1·66] vs 0·76 [0·68–0·86]; appendix pp 88–89). However, FEV1 postbronchodilator reversibility showed a non-linear relationship with future severe asthma attacks (Figure 3C). Notably, the common 12% reversibility (Figure 3D) was associated with some of the lowest adjusted attack rates, whereas risk was higher with reversibility greater than 25%. A sensitivity analysis excluding the 11 RCTs that required postbronchodilator reversibility at baseline (removing 4269 [66%] of 6513 participants), 12,15,16,32,34,36,40 and evaluating the prognostic value by FEV1% categories prebronchodilator and postbronchodilator, supported the overall negative prognostic effect of reversibility (appendix pp 43, 90). Reversibility was distributed similarly across asthma severities (appendix p 44).
The C-statistic for identification of a trial versus other trials ranged from 0·58 to 0·95 (appendix p 72), indicating major differences in patient and disease characteristics between studies. In the univariable meta-analysis per trial, we found substantial heterogeneity in associations between studies, with I2 statistics ranging from 0·56 to 0·97 (appendix p 15–35).
Discussion
In this large, IPD meta-analysis of control groups from RCTs across various clinical contexts, countries, and inclusion criteria, we showed that two biomarkers of type 2 airway inflammation—blood eosinophils and FeNO—provide valuable and synergistic incremental prognostic information regarding the risk of asthma attacks. The incremental relative risk associated with these biomarkers and key clinical prognostic factors implies that the absolute risk due to type 2 inflammation is greater in patients with additional clinical prognostic factors. Specifically, 10-fold increases in baseline blood eosinophil count and FeNO were each associated with a 1·4-fold higher risk of a severe asthma attack. Such substantial changes in these measurements reflect those that can occur with the initiation or discontinuation of an anti-inflammatory treatment. 14–16,30,42 These prognostic effects were greater than those associated with minimal clinically important differences in baseline FEV1 and ACQ-5. By contrast, after adjusting for type 2 biomarkers, moderate bronchodilator reversibility was associated with reduced risk of future asthma attacks.
The combination of blood eosinophils and FeNO might be more useful than either biomarker alone, since each reflects distinct components and compartments of the type 2 immune response in asthma. Blood eosinophil counts reflect circulating IL-5 and the systemic pool of available effector cells, whereas FeNO is an IL-13-mediated biomarker also reflecting type 2 cytokine, chemokine, and alarmin signalling in the airway compartment.9 Accordingly, elevations of both of these biomarkers are likely to be associated with migration of eosinophils to the bronchi and the development of airway pathology associated with asthma attacks, including abnormal mucus production, airway wall thickening, and increased bronchial motor tone.5,9,43 We previously found that patients with persistently elevated FeNO and blood eosinophil count had corticosteroid-resistant type 2 inflammation and refractory asthma despite adequate inhaled corticosteroid therapy.42 Moreover, airway mucus plugging in people with asthma has been linked to eosinophilia and greater airflow obstruction.43 Thus, raised type 2 biomarkers have substantial incremental utility to identify patients at risk. This finding is important as these measurements are known to identify patients who benefit most from anti-inflammatory therapies. 7,12–17
Our analyses focused on type 2 biomarkers while adjusting for clinical prognostic factors that are generally acknowledged as important. 1 By quantifying the multivariable prognostic values for inflammatory and clinical covariates, we found that the strongest increases in risk were associated with asthma attack history and treatment step (reflecting increased therapeutic intensity of asthma). Although these two cardinal variables are robust in identifying patients at risk, they do not reveal targetable mechanisms on the individual level. Similarly, other identified prognostic factors, such as symptoms and impaired lung function, can be modified independently of their effect on asthma attacks. Our results support the incremental value of biomarkers to improve risk stratification. Notably, the combination of blood eosinophils and FeNO was not part of clinical prediction models identified in a systematic review published in 2018.25 Surprisingly, bronchodilator reversibility, a common diagnostic tool and inclusion criterion for asthma trials, was associated with a reduced risk of asthma attacks. Overall, these findings have important implications for clinical practice as they highlight the shortcomings of relying on symptoms, lung function, and bronchodilator reversibility to identify patients at high risk and make individualised treatment decisions.1,5,44 Acknowledging that asthma attack history and disease severity had the greatest prognostic effects, further analyses can focus on understanding the interactions between type 2 inflammation and other clinical predictors in the dataset.
A notable strength of this meta-analysis compared with other prognostic studies in airway disease is the collaboration of academic, public, and pharmaceutical data providers, allowing efficient use of high-quality RCT IPD. Our study of the control groups of RCTs contrasts with previous prognostic analyses involving blood eosinophils and FeNO that were conducted in real-world settings or clinical trials in which background treatment fluctuated.45 The advantage of RCT data is that baseline prognostic factors and outcomes are assessed prospectively, with high confidence in diagnostic accuracy, treatment adherence, and stability. Furthermore, we adjusted our analyses for the heterogeneity observed between RCTs, improving the applicability of our findings across various clinical contexts, countries, and patient characteristics. In effect, the prognostic values we report in ORACLE2 are robust prospective estimates for a status quo scenario (ie, if treatment is unchanged following a clinical encounter).
We acknowledge that our study has limitations. First, our dataset comprised selected RCT populations that had lower asthma attack rates than expected due to regression to the mean and a placebo response. Nonetheless, we believe RCTs are a key high-quality source of evidence for prognostic factors because their control groups provide long-term clinical observation under stable background treatment. Second, we identified discrepancies between definitions of some variables in the included RCTs. These discrepancies improve the generalisability of the results as the prognostic effects remained consistent despite variations in study definitions. In keeping with best practices,23 we have disclosed our data dictionary, extraction code, and analytical code to ensure transparency and reproducibility of our study. Nevertheless, we acknowledge that patient-reported comorbidities, such as eczema, allergic rhinitis, airborne allergic sensitisation, and chronic rhinosinusitis with or without nasal polyposis, were unverified and inconsistently reported. 46 Accordingly, we downgraded the certainty of evidence for those characteristics’ prognostic effects. Third, the IPD meta-analysis included studies published up to April 1, 2021, which were identified through MEDLINE only. We were constrained in our ability to expand or update the systematic review after protocol development due to contracting requirements and practicalities. Fourth, we were unable to conduct analyses on social determinants of health, such as race, ethnicity, or socioeconomic status, due to the absence of standardisation for race and ethnicity across trials and the absence of data for socioeconomic status.1 Fifth, we quantified the risk attributable to type 2 inflammation using metrics adapted to the log-normal data distribution, and to maximise therapeutic relevance, we used 10-fold changes for the biomarkers to mirror the effects of initiation or discontinuation of an antiinflamma-tory treatment 14–16,30,42 and we estimated prognostic effects for absolute biomarker differences for pre-specified categories,18 interquartile cutpoints,41 and 2-fold changes. Finally, studies differed in patient profile and design. Although we included the full range of asthma severities, most patients had moderate-to-severe asthma, potentially due to the predefined exclusion of trials not measuring both blood eosinophils and FeNO. Notably, studies applied different control group formulations and schedules. Importantly, despite between-study differences in the selection of patients, intervention, and exact definitions of covariates, the prognostic effects were largely consistent across studies.
Our findings have substantial clinical implications. First, high-quality control group RCT IPD analyses show that inflammatory and clinical risk stratification should be combined to estimate risk on an individual basis. Second, our observation that blood eosinophil count and FeNO are synergistic prognostic factors in the context of the status quo scenario of RCT control groups (ie, no change in background therapy following assessment) has substantial implications for future risk prediction modelling.18 Indeed, one approach to targeted risk reduction is to conjugate risk stratification with mechanistic targeting (ie, to focus on prognostic factors that also predict benefits from specific preventive treatment).47 This approach has been successful in cardiovascular disease,4 in which the focus is on the effect of modifiable factors, such as blood pressure and cholesterol, on top of unmodifiable risk factors, such as age and sex. Just as meta-regression studies have shown that statin therapies target the modifiable risk of heart attacks in a biomarker-dependent way,48 the high asthma attack risk for people with type 2-high asthma in our dataset probably reflects the magnitude of the treatment opportunity. Specifically, we observed a higher annualised asthma attack rate for patients with high biomarkers than for those with low biomarkers, mirroring the previously reported treatment effect of higher-dose inhaled corticosteroids or biologics in biomarker-high asthma.7 These findings support the development of a framework analogous to that used in cardiovascular medicine to predict and prevent asthma attacks on an individual basis.47
In conclusion, blood eosinophil count, FeNO, asthma attack history, disease severity, lower lung function, and symptoms (ACQ-5 score) are key predictors of asthma attacks when treatment is unchanged. Importantly, high blood eosinophils and high FeNO together are associated with a greater risk than either factor alone, each highlighting specific treatment opportunities. 12–17,49 Moderate FEV1 postbronchodilator reversibility is associated with reduced risk of future asthma attacks. These findings underscore the importance of comprehensive risk stratification of people with asthma. Future prediction models using the ORACLE2 dataset could be centred on biomarkers for more individualised clinical decision making.
Contributors
FLM conducted data analyses and interpretation, verified the underlying data, and contributed to the first draft of the manuscript. SM-L, CC-P, SL-P, and SR contributed to data analysis. MEH, GB, JC, JH, SED, CEB, MC, NAH, DJJ, NM, AL, ES, CC, CTJH, AS, and MEW were primary investigators or employees of study sponsors of the underlying trials contributing IPD. TSCH contributed to data interpretation. RWB contributed to underlying trials, data interpretation, and study oversight. JKS and EWS contributed to data analysis, interpretation, and study oversight. IDP contributed to underlying trials, study preparation, data interpretation, and study oversight. SC set up the study, verified the underlying data, contributed to data collection, analysis, and interpretation, and is the guarantor of the study. FLM, EWS, IDP, and SC had access to the complete dataset. All authors read and approved the final draft of the manuscript and agreed to submit for publication.
Affiliations & Notes
a. Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, Netherlands b. Faculté de médecine et des sciences de la santé, Université de Sherbrooke, Sherbrooke, QC, Canada c. Centre de recherche du CHUS, CIUSSS de l’Estrie—CHUS, Sherbrooke, QC, Canada d. Institute for Respiratory Health, University of Western Australia, Perth, WA, Australia e. National Jewish Health, Denver, CO, USA f. Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium g. UCLA, Los Angeles, CA, USA h. North Bristol NHS Trust, Bristol, UK i. Department of Respiratory Sciences, Institute for Lung Health, NIHR Respiratory BRC, University of Leicester, Leicester, UK j. Pulmonary Critical Care & Sleep Medicine, University of Kansas, Kansas City, KS, USA k. Baylor College of Medicine, Houston, TX, USA l. Guy’s Severe Asthma Centre, Guy’s and St Thomas’ Hospitals, London, UK m. AstraZeneca, Cambridge, UK n. GlaxoSmithKline, London, UK o. Sanofi, Cambridge, MA, USA p. Genentech, San Francisco, CA, USA q. Novartis Healthcare Private, Hyderabad, India r. Respiratory Medicine Unit and Oxford Respiratory NIHR BRC, Nuffield Department of Medicine, University of Oxford, Oxford, UK s. Medical Research Institute of New Zealand, Wellington, New Zealand t. Julius Center, University Medical Center Utrecht, Utrecht, Netherlands
Data sharing statement
Data underlying each trial are available according to the terms in the original trial publications. The aggregate dataset generated by this study might be shared if the proposed use of the data has been approved by all original data providers. The external data extraction programming code was requested and made available on GitHub whenever possible (https://github.com/flmeulmeester/ ORACLE2/).
Declaration of interests
Outside this work, CC-P has received speaker honoraria from AstraZeneca, GSK, and Sanofi-Regeneron and consultancy fees from AstraZeneca, GSK, and Sanofi-Regeneron. SR has received salary support from the National Institute for Health and Care Research (NIHR) UK and the Charlie’s Foundation for Research. SR also declares speaker fees from GSK and AstraZeneca, and conference travel support from AstraZeneca. MEW has received consulting, advisory, or speaking honoraria from Allakos, Amgen, Areteia Therapeutics, Arrowhead Pharmaceutical, Astra-Zeneca, Avalo Therapeutics, Celldex, Connect Biopharma, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Kymera, Merck, MyBiometry, Pharming, Phylaxis, Pulmatrix, Rapt Therapeutics, Recludix Pharma, Regeneron, Roche/ Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Uniquity Bio, Upstream Bio, Verona Pharma, and Zurabio. GB has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Merck Sharp & Dohme, Novartis, and Sanofi-Regeneron; he is President of the Belgian Respiratory Society. JC has received grants or contracts from Regeneron, Sanofi, and Novartis. He has also received consulting fees from AstraZeneca, Amgen, Regeneron, and Sanofi and payment of honoraria from AstraZeneca, Amgen, Regeneron, and Sanofi. SED has received consultancy fees from AstraZeneca. CEB has received grants and consultancy fees from 4D Pharma, Areteia, AstrZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche, and Sanofi. MC has received grants or contracts from the American Lung Association, AstraZeneca, Gala Therapeutics, Genentech, GSK, National Institutes of Health, Nocion, Novartis, PCORI, Pulmatrix, Sanofi-Aventis, Shionogi, and Theravance Biopharma. He has also received consulting fees from Allakos, Amgen, Apogee, Apreo Health, Arrowhead Pharmaceuticals, Blueprint Medicines, Connect BioPharma, Evommune, Genentech, GSK, Jasper, Kinaset, Merck, Novartis, OM Pharma, Pfizer, Pioneering Medicines, Sanofi-Aventis, Teva, Third Rock Ventures, Upstream Bio, and Verona Pharmaceuticals; honoraria from Amgen, AstraZeneca, Med Learning Group, Regeneron Pharmaceuticals, and Sanofi; and stock options from Aer Therapeutics. NAH has received honoraria for serving as a consultant or advisor to GSK, AstraZeneca, Genentech, Sanofi, Regeneron, Verona, and Amgen; and research grant support from GSK, AstraZeneca, Genentech, Regeneron, and Sanofi. DJJ has received advisory board and speakers’ fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva, GSK, Sanofi-Regeneron, and Chiesi. NM is an employee and shareholder with AstraZeneca. AL is employed by AstraZeneca. ES is a former GSK employee and provided anonymised data from GSK studies CAPTAIN and DREAM; provided inputs into manuscript development; and holds GSK stock options. CC holds shares in GSK and is an employee of GSK. MEH is a Sanofi employee. CTJH is a former employee of Genentech. AS is a Novartis employee. TSCH was supported by a Wellcome Trust Fellowship (211050/Z/18/z); he reports grants from the Guardians of the Beit Fellowship, Pfizer, NIHR Oxford Biomedical Research Centre (BRC), University of Oxford, Kymab, Arcturis, and Asthma+Lung UK; and personal fees from AstraZeneca Pieris. RWB has received institutional research funding from AstraZeneca, Teva, Health Research Council, Cure Kidz, and Perpetual Guardian; personal fees from AstraZeneca, Avillion, and Teva; and is Chair of the Asthma Foundation of New Zealand adolescent and adult asthma guidelines, a reviewer for GINA, and a former member of the Global Initiative for Chronic Obstructive Lung Disease board. JKS has received non-restricted research grants from AstraZeneca, European Respiratory Society Severe Heterogeneous Asthma Research collaboration—Patient Centred Clinical Research Collaboration, Register of Adult Patients with Severe Asthma for Optimal Disease Management Foundation, and ZonMw. EWS has received consultancy fees from GSK. IDP has received honoraria for speaking at sponsored meetings from Astra-Zeneca, Circassia, AmgenNovartis, Chiesi, Sanofi-Regeneron, Menarini, and GSK; and payments for organising educational events from AstraZeneca, GSK, Sanofi-Regeneron, and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi-Regeneron, AstraZeneca, GSK, Novartis, Teva, Merck, Circassia, and Amgen. He has received sponsorship to attend international scientific meetings from GSK, AstraZeneca, Sanofi, and Regeneron. SC reports non-restricted research grants from NIHR Oxford BRC, the Quebec Respiratory Health Research Network, the Fondation Québécoise en Santé Respiratoire, AstraZeneca, Sanofi-Regeneron, and Circassia Niox group; speaker honoraria from AstraZeneca, GSK, Sanofi-Regeneron, and Valeo Pharma; consultancy fees from FirstThought, AstraZeneca, GSK, Sanofi-Regeneron, Access Biotechnology, and Access Industries; and sponsorship to attend or speak at international scientific meetings by or for AstraZeneca and Sanofi-Regeneron. He is an advisory board member for and holds stock options in Biometry, a company that is developing an exhaled nitric oxide device (myBiometry). He advised the Institut national d’excellence en santé et services sociaux on an update of the asthma general practice information booklet for general practitioners. Within the submitted work, CC-P has received an education scholarship from the Université de Sherbrooke, and SC reports that he has received non-restricted research grants from the Québec Air-Intersectorialité-Respiratoire-Son network, the Academy of Medical Sciences, and the NIHR Oxford BRC, is the holder of the Association pulmonaire du Québec’s Research Chair in Respiratory medicine, and is a clinical research scholar of the Fonds de recherche du Québec. All other authors declare no competing interests.
Acknowledgments
We thank Arianne Caron and Rachel Russel-Sharpe for administrative support, Jack Howarth for legal expertise, Lars van der Burg and Edouard Bonneville for statistical expertise and support, André Carpentier, André Cantin, and Xavier Jamont (former Novartis employee) for feedback on the manuscript, and our patients for continued inspiration and motivation to advance knowledge in asthma. This work was supported by the NIHR Oxford BRC, Association pulmonaire du Québec, Fonds de recherche du Québec—Santé, Québec Air-Intersectorialité-Respiratoire-Son network, Stichting Astma Bestrijding, Leiden University Fund, and Academy of Medical Sciences. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, the Department of Health, or the other funders.
References
1. Global Initiative for Asthma Global strategy for asthma management and prevention https://gin asthma. org/wp-content/uploads/2024/ 05/GINA-2024-Strategy-Report-24_05_22_ WMS.pdf
2. Couillard, S ∙ Petousi, N ∙ Smigiel, K ∙ et al. Towards a predictive and preventive approach in obstructive airway diseases. J Allergy Clin Immunol Pract. 2023; 11:704-712
3. Lazarus, SC ∙ Boushey, HA ∙ Fahy, JV et al. Long-acting β2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomised controlled trial
4. Conroy, RM ∙ Pyörälä, K ∙Fitzgerald, AP ∙ et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003; 24:987-1003
5. Pavord, ID ∙ Beasley, R ∙ Agusti, A ∙ et al. After asthma: redefining airway diseases. Lancet. 2018; 391:350-400
6. Couillard, S ∙ Laugerud, A ∙ Jabeen, M ∙ et al. Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide. Thorax. 2022; 77:199-202
7. Couillard, S ∙ Do, WIH ∙ Beasley, R ∙ et al. Predicting the benefits of type-2 targeted anti-inflammatory treatment with the prototype Oxford Asthma Attack Risk Scale (ORACLE) ERJ Open Res. 2021; 8:1-5
8. Celis-Preciado, C ∙ Leclerc, S ∙ Duval, M ∙ et al. Phenotyping the responses to systemic corticosteroids in the management of asthma attacks (PRISMA) Eur Respir J. 2025; published online Feb 13. https:// doi.org/10.1183/13993003. 02391- 2024
9. Couillard, S∙ Shrimanker, R∙ Chaudhuri, R ∙ et al. Fractional exhaled nitric oxide nonsuppression identifies corticosteroid-resistant type 2 signalling in severe asthma. Am J Respir Crit Care Med. 2021;204: 731-734
10. Beasley, R ∙ Holliday, M ∙ Reddel, HK et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019; 380:2020-2030
11. Pavord, ID ∙ Holliday, M ∙ Reddel, HK et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med. 2020; 8:671-680
12. Lee, LA ∙ Bailes, Z ∙ Barnes, N ∙ et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021; 9:69-84
13. Couillard, S ∙ Pavord, ID. Fluticasone furoate: CAPTAIN of fluticasones in type 2 inflammatory asthma. Respirology. 2022; 27:184-186
14. Pavord, ID ∙ Korn, S ∙ Howarth, P ∙ et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651-659
15. Castro, M ∙ Corren, J ∙ Pavord, ID ∙ et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018; 378:2486-2496
16. Menzies-Gow, A∙ Corren, J ∙ Bourdin, A ∙ et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma N Engl J Med. 2021; 384:1800-1809
17. FitzGerald, JM∙ Bleecker, ER ∙ Menzies-Gow, A∙ et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018; 6:51-64
18. Couillard, S ∙ Steyerberg, E ∙ Beasley, R ∙ et al. Blood eosinophils, fractional exhaled nitric oxide and the risk of asthma attacks in randomised controlled trials: protocol for a systematic review and control arm patient-level meta-analysis for clinical prediction modelling BMJ Open. 2022; 12, e058215
19. Stewart, LA ∙ Clarke, M ∙ Rovers, M ∙ et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement JAMA. 2015; 313: 1657-1665
20. Sauerbrei, W ∙ Taube, SE∙ McShane, LM∙ et al. Reporting recommendations for tumour marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst. 2018; 110:803-811
21. Hardy, J ∙ Baggott, C ∙ Fingleton, J ∙ et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019; 394:919-928
22. Higgins, J ∙ Thomas, J ∙ Chandler, J ∙ et al. Cochrane handbook for systematic reviews of interventions (version 6.2). https://training. cochrane.org/handbook
23. Huebner, M ∙ Vach, W ∙ le Cessie, S ∙ et al. Hidden analyses: a review of reporting practice and recommendations for more transparent reporting of initial data analyses. BMC Med Res Methodol. 2020; 20:61
24. White, IR ∙ Royston, P ∙ Wood, AM Multiple imputation using chained equations: issues and guidance for practice Stat Med. 2011; 30:377-399
25. Loymans, RJB∙ Debray, TPA ∙Honkoop, PJ ∙ et al. Exacerbations in adults with asthma: a systematic review and external validation of prediction models. J Allergy Clin Immunol Pract. 2018; 6:1942-1952
26. Parker, RA∙ Weir, CJ. Non-adjustment for multiple testing in multi-arm trials of distinct treatments: rationale and justification. Clin Trials. 2020; 17:562-566
27. Langendam, MW ∙ Akl, EA ∙ Dahm, P ∙ et al. Assessing and presenting summaries of evidence in Cochrane Reviews Syst Rev. 2013; 2:81
28. Brusselle, GG ∙ Vanderstichele, C ∙ Jordens, P ∙ et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68:322-329
29. Park, HS ∙ Kim, MK ∙ Imai, N ∙ et al. A phase 2a study of benralizumab for patients with eosinophilic asthma in South Korea and Japan Int Arch Allergy Immunol. 2016; 169:135-145
30. Castro, M ∙ Wenzel, SE ∙ Bleecker, ER ∙ et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879-890
31. Harris, JM ∙ Maciuca, R ∙ Bradley, MS ∙ et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res. 2016; 17:29
32. Wenzel, S ∙ Castro, M ∙ Corren, J ∙ et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016; 388:31-44
33. Hanania, NA ∙ Wenzel, S ∙ Rosén, K ∙ et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804-811
34. Hanania, NA∙ Korenblat, P∙ Chapman, KR ∙ et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016; 4:781-796
35. Brightling, CE ∙ Gaga, M ∙ Inoue, H ∙ et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir Med. 2021; 9:43-56
36. Hanania, NA ∙ Noonan, M ∙ Corren, J ∙ et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015; 70:748-756
37. Corren, J ∙ Lemanske, Jr, RF ∙ Hanania, NA ∙ et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088-1098
38. Sorkness, CA ∙ Lemanske, Jr, RF∙ Mauger, DT ∙ et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the pediatric asthma controller trial. J Allergy Clin Immunol. 2007; 119:64-72
39. Corren, J ∙ Parnes, JR ∙ Wang, L ∙ et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017; 377:936-946
40. Panettieri, Jr, RA ∙ Sjöbring, U ∙ Péterffy, A ∙ et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med. 2018; 6:511-525
41. Steyerberg, EW. Clinical prediction models. A practical approach to development, validation, and updating. Springer, Cham, 2019
42. Couillard, S∙ Shrimanker, R∙ Lemaire-Paquette, S ∙ et al. Longitudinal changes in sputum and blood inflammatory mediators during FeNO suppression testing, Thorax. 2022; 77:933-938
43. Dunican, EM ∙ Elicker, BM ∙ Gierada, DS ∙ et al. Mucus plugs in patients with asthma are linked to eosinophilia and airflow obstruction, J Clin Invest. 2018; 128:997-1009
44. Beasley, R ∙ Hughes, R ∙ Agusti, A ∙ et al. Prevalence, diagnostic utility and associated characteristics of bronchodilator responsiveness. Am J Respir Crit Care Med. 2024; 209:390-401
45. Gruchalla, RS ∙ Sampson, HA ∙ Matsui, E ∙ et al. Asthma morbidity among inner-city adolescents receiving guideline-based therapy: The role of predictors in the setting of high adherence. J Allergy Clin Immunol. 2009; 124:213-221
46. Fokkens, WJ ∙ Lund, VJ ∙ Hopkins, C ∙ et al. European position paper on rhinosinusitis and nasal polyps 2020 Rhinology. 2020; 58:1-464
47. Kent, DM ∙ Steyerberg, E ∙ van Klaveren, D Personalised evidence-based medicine: predictive approaches to heterogeneous treatment effects BMJ. 2018; 363, k4245
48. Baigent, C ∙ Blackwell, L ∙ Emberson, J ∙ et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670-1681
49. Couillard, S ∙ Jackson, DJ ∙ Pavord, ID∙ et al. Choosing the right biologic for the right patient with severe asthma. Chest. 2025; 167:330-342
Credits: Meulmeester Fleur L, Mailhot-Larouche Samuel, Celis-Preciado Carlos, Lemaire-Paquette Samuel, Ramakrishnan Sanjay, Wechsler Michael E, Brusselle Guy, Corren Jonathan, Hardy Jo, Diver Sarah E, Brightling Christopher E, Castro Mario, Hanania Nicola A, Jackson David J, Martin Neil, Laugerud Annette, Santoro Emilio, Compton Chris, Hardin Megan E, Holweg Cecile T J, Subhashini Allu, Hinks Timothy S C, Beasley Richard W, Sont Jacob K, Steyerberg Ewout W, Pavord Ian D, Couillard Simon. Inflammatory and clinical risk factors for asthma attacks (ORACLE2): a patient-level meta-analysis of control groups of 22 randomised trials. The Lancet Respiratory Medicine, Elsevier, 2025; 2213-2600. https://doi. org/10. 1016/S2213-2600(25)00037-2