Okonkwo Uchenna C1*, Aluka Anthony2 and Ezedinachi Emmanuel1
1Department of Internal Medicine, University of Calabar Teaching Hospital, Calabar, Nigeria
2Department of Family Medicine, University of Calabar Teaching Hospital, Calabar, Nigeria
*Corresponding author: Dr. Okonkwo UC, Department of Internal Medicine, University of Calabar Teaching Hospital, P.M.B. 1278, Calabar, Cross river state, Nigeria. Tel: +234 803 3251 240; Email: drucokonkwo@unical.edu.ng
Abstract
Background: Silymarin, an extract of milk thistle (Silybum marianum) is widely used in the treatment of patients with chronic liver disease yet its beneficial effects remain controversial.
Aim: To determine the effect of silymarin on liver biochemistry and health-related quality of life (HRQOL) in treatment naïve patients with chronic hepatitis B infection.
Methods: This was a double-blind randomized placebo-controlled trial conducted at the University of Calabar
Teaching Hospital, Cross river state. Patients with chronic hepatitis B were randomized to receive silymarin or placebo for 4 weeks. Their liver function tests and HRQOL were assessed at baseline and post-treatment.
Results: Eighty-six persons were enrolled in the study. Seventy-nine completed the study. Silymarin use was associated (p<0.05) with improvement in serum alanine and aspartate aminotransferase and HRQOL from baseline but not with serum bilirubin and alkaline phosphatase.
Conclusion: Silymarin use among patients with chronic hepatitis B is associated with improvement in liver function tests especially markers of liver inflammation and HRQOL.
Keywords: Chronic hepatitis B; Silymarin; Randomized controlled trial
Introduction
Current treatment of chronic viral hepatitis B with interferon and nucleoside analogs has remained unsatisfactory with seroconversion of hepatitis B virus from a replicative to a non-replicative state occurring in only 15-32% of patients 1,2. Along with this high rate of treatment failure, is the prohibitive cost of these medications especially interferon-based medications, and associated numerous side effects. This is especially true in Nigeria where out-of-pocket payment for healthcare services is still very much the rule. Many patients are therefore too eager to explore the use of complementary and alternative treatment with their optimistic and somewhat sketchy evidence of benefit.
Silymarin, an extract of the milk thistle herb (Silybum marianum), has been in use for the treatment of liver diseases since ancient times. In the 15th century, Nicholas Culpepper, an English herbalist described the milk thistle as being good against jaundice 3. Silymarin is composed of flavonolignans namely silybin, silydianin, silycristin as well as a diastereomer of silybin; isosilybin. Most of its hepatoprotective effect is attributed to silybin which constitutes 60-70% of the drug 4. It is poorly absorbed in the gastrointestinal tract and primarily excreted in bile.
Silymarin is the most commonly consumed complementary and alternative medicine reported in patients with chronic hepatitis 5. A study in the USA found the prevalence of the use of herbal medications for the treatment of allied medical conditions to have increased from 2.5% to 12.1% between 1990 and 1997 6. Another study in the same country reported that as much as 31% of patients attending a hepatology clinic in Oregon are using alternative remedies such as milk thistle 7.
Silymarin is thought to exert its beneficial effects in chronic liver diseases because of its membrane-stabilizing properties. It promotes hepatocyte regeneration by stimulating nucleolar polymerase A and increasing ribosomal protein synthesis. Silybin selectively inhibits leukotriene formation by Kupffer cells and also exhibits some antioxidant properties by increasing superoxide dismutase activity in the red cells 8,9. In addition, it has immune-modulatory effects and enhances the secretion of interferon-gamma, interleukin 4, and 10 10. Thus, it has been shown to lead to the normalization of serum aminotransferases in patients with chronic liver diseases especially chronic hepatitis C virus infection where it has been documented to prevent graft re-infection after orthotopic liver transplantation in patients with decompensated chronic hepatitis C infection by inhibiting entry of hepatitis C viral particles into transplanted liver 11,12.
Silymarin and its components have shown beneficial effects in reversing liver injury secondary to chronic alcohol abuse and drugs/poisons such as Amanita phalloides (deadly mushroom) 13-15. Few studies have been done to evaluate the effect of this medication in patients with predominantly hepatitis B-associated chronic liver disease 14,15 and none has been done in Nigeria. Hence, this study was designed to evaluate the effects of silymarin on liver biochemistry and quality of life in patients with chronic viral hepatitis B infection and compare this to the effect of a placebo.
General Objective
To determine the effect of silymarin on liver biochemistry and quality of life in treatment naïve patients with chronic viral hepatitis B infection after one month of treatment and compare it to placebo.
Specific Objectives
1. To evaluate the effect of silymarin on liver function tests (bilirubin, aspartate amino transaminase (AST), alanine amino transaminase (ALT), and alkaline phosphatase (ALP).
2. To determine the effect of silymarin on health-related quality of life (HRQOL).
Subjects, Materials, and Methods
This was a double-blind randomized, placebo-controlled study carried out at the Gastroenterology clinic and the Family Medicine clinic of the University of Calabar Teaching Hospital, Calabar. It lasted from January-December, 2013. The study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the University of Calabar Teaching Hospital Ethical Board. All participants gave written informed consent. A sample size of 84 was calculated using the formula13 N=f ((α,p)x2xδ2 /d2. Where δ is the postulated difference observed in the two groups=4, d is the standard error of the sample difference =2, p is the power of the test=90% and α is the level of significance=5%
Adult patients who were 18 years and above with a diagnosis of chronic hepatitis B viral infection were randomized to receive silymarin manufactured by Micro Labs Limited, India, and marketed as Silybon by Micro-Nova Pharmaceuticals Plc in Nigeria or placebo for a period of four weeks. The active ingredients and placebo were placed in identical tablets as the commercial product and provided in bulk by Micro Nova Pharmaceuticals. Inclusion criteria included a positive HBsAg result for 6 months or more and the presence of stigmata of chronic liver disease such as leuconychia, jaundice, and liver palms. A liver biopsy was not part of the diagnostic criteria for this study. Exclusion criteria were being HBsAg negative, presence of features of liver decompensation such as ascites, hepatic encephalopathy, variceal bleeding, and history of past or current use of anti-viral drugs such as interferon or any of the nucleoside/nucleotide analogues.
At first contact, a structured questionnaire was used to document
demographic information, symptoms, signs, and risk of chronic liver disease including use of herbal remedies. The SF36 questionnaire on health-related quality of life (HRQOL) was also administered. Five milliliters of venous blood was collected from the antecubital fossa observing the universal precautionary measures for evaluation of liver function tests. The patients were randomized to receive silymarin or a placebo using pre-selected random numbers folded in a paper. Both investigators and patients were blinded to which tablet contained the active ingredient or placebo. At the end of four weeks, the HRQOL questionnaire was re-administered and blood was collected to repeat liver function test. Patients who failed to show up after four weeks had a reminder in the form of a phone call or text message. They were considered to have dropped out if they fail to come within two weeks afterward.
Statistical Analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS) version 15. Continuous variables were tested for normal distribution and expressed as mean ± Standard deviation or median as appropriate. Categorical variables were compared by Pearson’s chi-square test. Mean values of bilirubin, AST, ALT, and ALP at baseline were compared with post-treatment values using the paired sample t-test. Scores for the SF36 questionnaire were calculated online at https://www. amihealthy.com/Surveys/sf36 Pre and post-treatment scores were also compared using the paired sample t-test. A p-value of <0.05 was considered significant.
Results
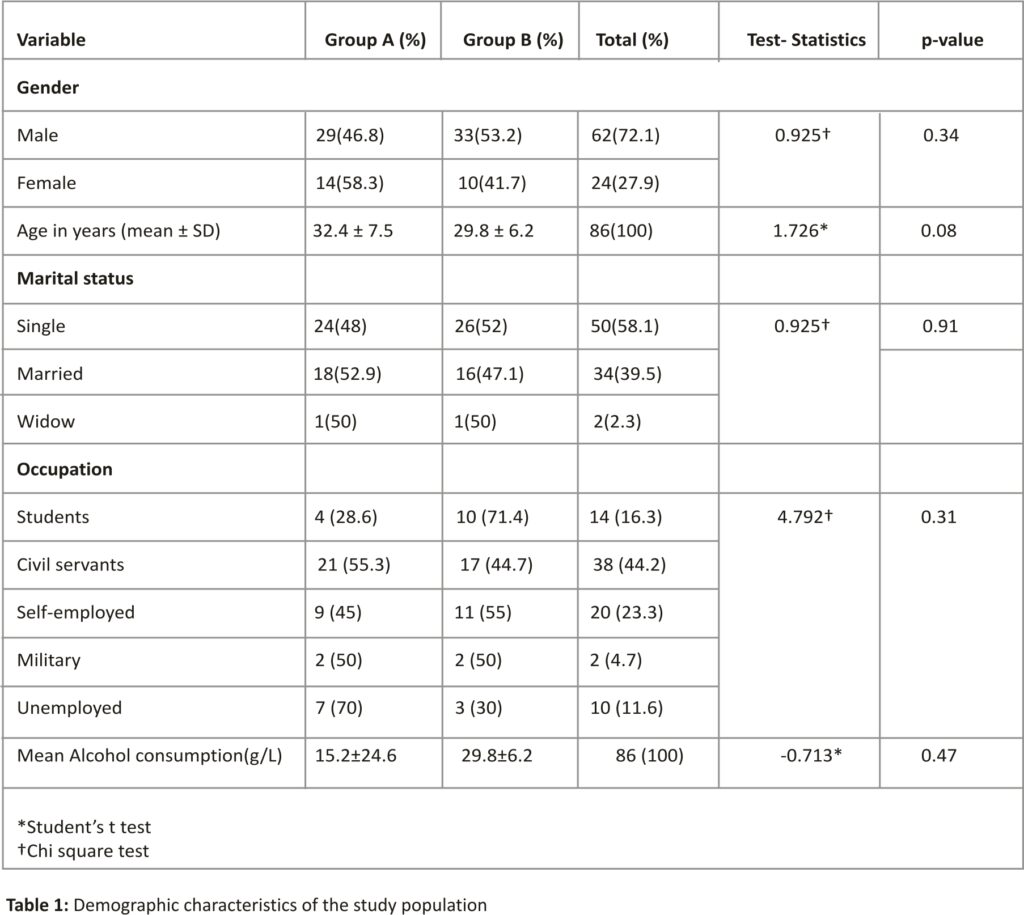
Eighty-six consecutive patients with chronic hepatitis B infection were randomized to receive 140 mg of silybon three times daily or placebo for 4 weeks. There were 62 males and 24 females. The M: F ratio was 2.6:1. Their ages ranged from 18-54 years with a mean age of 31.15 ± 7 years. In group A (silybon), there were 29 males and 14 females while in group B(placebo), there were 33 males and 10 females The demographic characteristics of groups A and B were not statistically significant (p>0.05) (Table 1).
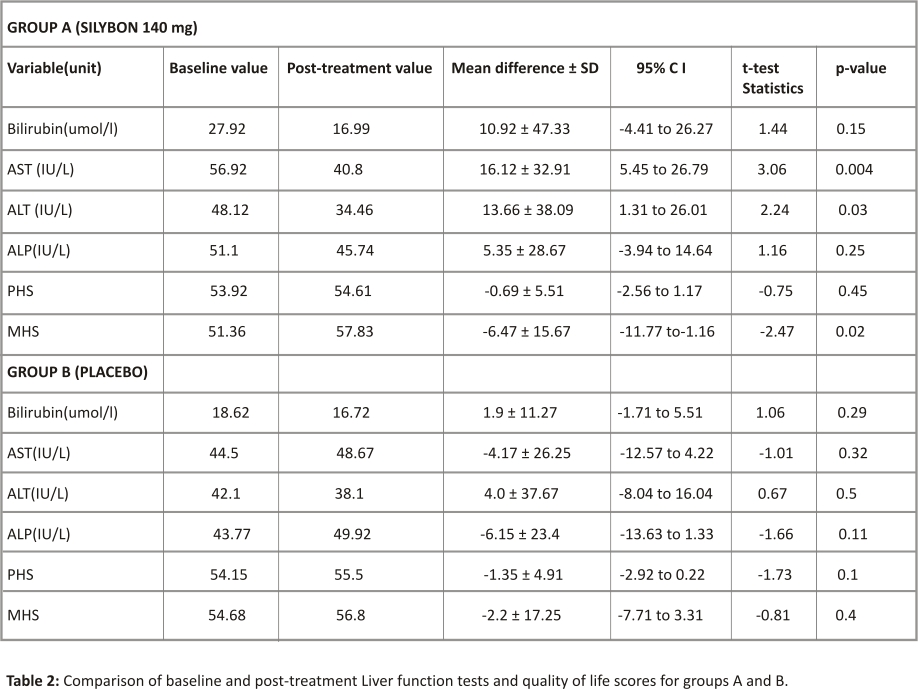
Seventy-nine persons completed the study. Seven persons dropped out of the study. Mild adverse event (pruritus, headache, epigastric discomfort, and throat irritation) was the reason for dropping out in 5 persons. Three in the treatment group and 2 in the placebo group. While in 2 persons, the reason for dropping out was not documented. In group A, 39 persons completed the study while 40 persons completed it in group B. The pre-treatment (baseline) values of the liver enzymes (Bilirubin, AST, ALT, ALP) when compared between the two groups was higher in group A but the difference was not statistically significant. In group A, the mean serum concentration of liver enzymes was reduced from baseline to post-treatment values and this reduction was statistically significant for AST and ALT but not for bilirubin and ALP. The percentages of the subjects whose LFT parameters reduced from baseline values were as follows; bilirubin (24/39=61.5%), AST(28/39= 71.8%), ALT(27/39=69.2%), and ALP(22/39=56.4%). In group B, the mean serum concentrations of liver enzymes were also reduced from baseline to post-treatment values except for AST and ALP. However, the differences were not statistically significant (p>0.05) respectively. The percentages of subjects who reduced their LFTs from baseline values were 47.5% for bilirubin, AST, and ALT respectively, and 45% for ALP. The physical health-related score and the mental health-related score showed some improvement in both groups. However, only the MHS score in group A showed statistically significant improvements (p=0.02) (Table 2)
The below table compares the baseline and post-treatment values of LFTs for groups A and B.
Discussion
Seventy-nine patients with chronic hepatitis B were randomized to receive silymarin 140 mg three times a week for 4 weeks or placebo for the same duration. All had their HRQOL assessed using the SF-36 questionnaire before and after the intervention. Our study showed that silymarin was generally safe and well tolerated as no major adverse event was reported and is in keeping with reports from other studies 14-19. There are compelling data in the literature on the beneficial effects of silymarin on liver injury caused by hepatotoxins such as alcohol and the deadly mushroom, Amanita phalloides 13-15.
Although some studies have shown that silymarin and its components improve liver function and hepatic fibrosis markers in patients with chronic hepatitis (either B or C) 20,21, others had refuted this claim 22. This may be because some of these studies focused on end-points such as liver cirrhosis and liver-related complications and mortality that are not affected by silymarin. The beneficial effect of silymarin on the liver is postulated to be due to its anti-oxidant and free radical scavenging properties. In addition, it promotes hepatocyte membrane stabilization, and protein synthesis and also modulates the immune response.
Our study showed that silymarin significantly reduced serum aspartate amino transaminase, and alanine aminotransaminase but not bilirubin and alkaline phosphatase from baseline. This is in keeping with findings by Bruzelli et al. 20 in their study of 20 patients with chronic hepatitis B or C. In a recent meta-analysis of the effects of silymarin or its combination in the treatment of chronic hepatitis B, it was shown that silymarin reduced serum transamianases16. Another study amongst patients with chronic hepatitis C found statistically significant improvements in serum AST, ALT, and quality of life scores amongst patients treated with silymarin 23. The mechanism of liver injury in chronic hepatitis B and C infection is largely immune-mediated and related to chronic inflammatory hepatocyte injury. The tendency for patients receiving silymarin to show better improvement in liver function is likely due to its free radical scavenging and membrane-stabilizing properties. Thus, it protects neighboring healthy hepatocytes from toxins released by damaged hepatocytes thereby reducing inflammation and systemic inflammatory responses that impact negatively on quality of life. This study demonstrated an improvement in quality of life scores in patients with chronic hepatitis B. The SF-36 questionnaire provides a psychometrically based physical and mental component summary. Patients with chronic hepatitis B are mostly asymptomatic or may have symptoms such as mild right hypochondria pain and fatigue. These do not cause limitations in performing physical activities. However, they are usually anxious about their health situation because of the misconception about the disease. This can impact negatively their mental health. Studies have shown that silymarin is associated with improved perception of well-being and quality of life scores in patients with acute and chronic viral hepatitis 23,24. It is been shown that quality of life is an important determinant of outcome in any type of chronic illness and many believe it is the single most important
factor in the recovery from chronic illnesses 25,26.
Many patients in Nigeria consume silymarin either on prescription or self-medication for a wide variety of liver diseases. Our study showed that a dose of 420 mg daily is safe and well tolerated and associated with some improvement in liver function and quality of life scores. Larger studies are needed to verify these effects. Future studies should incorporate end-points such as viral load and fibrosis markers and also evaluate effects on other aetiologies of liver disease.
Summary:
Chronic hepatitis B infection is highly prevalent in Nigeria. Standard therapy with interferon or nucleotide/nucleoside analogue is expensive and sometimes disappointing. Patients with chronic hepatitis B resort to the use of silymarin; a complementary and alternative medicine used in the management of liver diseases either on self or prescription medication. Its usefulness in chronic hepatitis B infection is contentious and has not been studied in a Nigerian population. Findings from this study hope to provide evidence-based data on its efficacy and possible side effects.
Acknowledgment
This research was supported by Micronova Pharmaceuticals. Manuscript preparation: The funding agency either directly or through a third party, had no role in the study design, gathering, preparation, or analyzing the data or in the writing of the manuscript. : Okonkwo U.C. and Aluka A designed the trial and collected data. Okonkwo U.C. contributed data analysis and manuscript writing. Ezedinachi E supervised manuscript writing. All approved final manuscripts.
References
1. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, et al. (2005) Peginterferon Alfa-2a, lamivudine, and the combination for HBsAg positive chronic hepatitis B. N Engl J Med 352: 2682-2695.
2. Conjeevaram HS, Lok AS (2003) Management of chronic hepatitis B. J Hepatol 38 Suppl 1: S90-103.
3. Karayiannis P (2003) Hepatitis B virus: old, new and future approaches to antiviral treatment. J Antimicrob Chemother 51: 761-785.
4. Wellington K, Jarvis B (2001) Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs 15: 465-489.
5. Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, et al. (2002) Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol 97: 2391-2397.
6. Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280: 1569-1575.
7. Flora K, Hahn M, Rosen H, Benner K (1998) Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol 93: 139-143.
8. Varga Z, Czompa A, Kakuk G, Antus S (2001) Inhibition of the superoxide anion release and hydrogen peroxide formation in PMNLs by flavonolignans. Phytother Res 15: 608-612.
9. Dehmlow C, Erhard J, de Groot H (1996) Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology 23: 749-754.
10. Wilasrusmee C, Kittur S, Shah G, Siddiqui J, Bruch D, et al. (2002) Immunostimulatory effect of Silybum Marianum (milk thistle) extract. Med Sci Monit 8: BR439-443.
11. Neumann UP, Biermer M, Eurich D, Neuhaus P, Berg T (2010) Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin mono-therapy. J Hepatol 52: 951-952.
12. Beinhardt S, Rasoul-Rockenschaub S, Maieron A, PH S-M, Hofer H PF (2012) Intravenous Silibinin-therapy in patients with chronic hepatitis C in the transplant setting. J Hepatology 56: S77.
13. Pares A, Planas R, Torres M, Caballeria J, Viver JM, et al. (1998) Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol: 615-621.
14. Rambaldi A, Jacobs BP, Iaquinto G, Gluud C (2005) Milk thistle for alcoholic and/or hepatitis B or C liver diseases–a systematic cochrane hepato-biliary group review with meta-analyses of randomized clinical trials. Am J Gastroenterol 100: 2583-2591.
15. Enjalbert F, Rapior S, Nouguier-Soulé J, Guillon S, Amouroux N, et al. (2002) Treatment of amatoxin poisoning: a 20-year retrospective analysis. J Toxicol Clin Toxicol 40: 715-757.
16. Wei F, Liu SK, Liu XY, Li ZJ, Li B, et al. (2013) Meta-analysis: silymarin and its combination therapy for the treatment of chronic hepatitis B. Eur J Clin Microbiol Infect Dis 32: 657-669.
17. Mayer KE, Myers RP, Lee SS (2005) Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat 12: 559-567.
18. Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA (2002) Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med 113: 506-515.
19. [No authors listed] (1999) An adverse reaction to the herbal medication milk thistle (Silybum marianum). Adverse Drug Reactions Advisory Committee. Med J Aust 170: 218-219.
20. Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, et al. (1993) A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol 31: 456-460.
21. Marcelli R, Bizzoni P, Conte D, Lisena MO, Lampertico M, et al. (1992) Randomized controlled study of the efficacy and tolerability of a short course of IdB 1016 in the treatment of chronic persistent hepatitis. European Bulletin of Drug Research1: 131-135.
22. Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, et al. (2012) Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA 308: 274-282.
23. Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, et al. (2011) Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment Pharmacol Ther 33: 127-137.
24. El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, et al. (2009) A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine 16: 391-400.
25. Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, et al. (2008) Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology 47:605-612.
26. Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, et al. (2006) Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol 21: 275-280.
Credits: Okonkwo Uchenna C, Aluka Anthony, Ezedinachi Emmanuel (2014) Effects of Silymarin on Treatment Naïve Patients with Chronic Hepatitis B Infection-A Randomized Controlled Trial. J Infect Dis Ther 2: 168. doi:10.4172/2332-0877.1000 168