MaryJoy Umoke, PhD,1 Peter Sage, PhD,2 Tor Bjoernsen, BSc,3 Prince Christian Ifeanachor Umoke, PhD,4 Christian Ezeugworie, MBBS,3 Daniel Ejiofor, BSc,3 Ogbonna Agha, BSc,3 Chioma Adaora Nwalieji, BSc,1 Rosemary N. Onwe, BSc,1 Ifeanyi Emmanuel Nwafor, MSc,5 and Obinna Jude Chukwu, MBBS5
1Ebonyi State Ministry of Health Abakaliki, Abakaliki, Ebonyi, Nigeria
2AMURT Global Coordinating Office, Washington, DC, USA
3AMURT, Abakaliki, Ebonyi, Nigeria
4University of Nigeria, Nsukka, Enugu, Nigeria
5Alex Ekwueme Federal University Teaching Hospital Abakaliki, Abakaliki, Ebonyi, Nigeria
Correspondence: MaryJoy Umoke, Ebonyi State Ministry of Health Abakaliki, Ishieke Campus, Abakaliki, Ebonyi PMB 053, Nigeria. Email: maryjoy4umoke@gmail.com
Abstract
Globally, sexually transmitted infections are recognized as a public and reproductive health challenge. The study determined the prevalence, co-infection, and risk factors associated with HBV, HCV, HIV, and Syphilis infections among pregnant women receiving antenatal care in rural health facilities in Ebonyi State, Nigeria. A retrospective study was conducted from January to December 2018 in 8 primary healthcare facilities using antenatal records of all the 4657 pregnant women who attended ANC within the period. Data were analyzed using descriptive and inferential statistics with IBM SPSS statistics version 20 and hypotheses tested at P < .05. The findings indicated a medium prevalence of HBV (4.1%), a high prevalence of HCV (4.1%) and syphilis (1.8%), and a low prevalence of HIV (0.9%). An overall co-infection rate of 0.623% that was not significant (P > .05) was observed. Also, the prevalence was more among the younger mothers (<20 years), those with secondary education. And the history of blood transfusion was significantly associated with HBV and HCV prevalence (χ2 = 7.865; P = .05*), 11.8%. conclusively, due to medium HBV prevalence and a high prevalence of HCV and syphilis observed, attention should be paid to blood screening before transfusion by health workers. Relevant stakeholders should provide intensive health education and appropriate free treatment services particularly for younger mothers and the less educated.
Keywords: prevalence, co-infection, STIs, pregnancy, Nigeria
What do we already know about this topic?
Reports are mainly on HIV and HBV among pregnant mothers but less on HCV and Syphilis,
How does your research contribute to the field?
Our research has gone further by juxtaposing HIV, HBV, HCV, and Syphilis infections in pregnant women,
What are your research’s implications toward theory, practice, or policy?
Pregnant mothers are tested for the infections but not treated except they pay for it thus there should be a policy that every pregnant mother tested to be given free treatment.
Introduction
Maternal, reproductive, and child health have been wrecked by sexually transmitted infections (STIs) in all countries of the world and the burden of the problem is more in many developing countries, including Nigeria.1 STIs have been globally recognized as major public and reproductive health challenges2,3 which can result to adverse pregnancy outcomes such as stillbirth, neonatal death, intrauterine growth retardation, premature rupture of the membrane, preterm birth, low birth weight and prematurity, congenital deformities, infant pneumonia, blindness among other complications if either poorly treated or entirely left untreated.2-5 Some common STIs include those from Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), Syphilis, genital herpes, Neisseria gonorrhoeae, Trichomonas vaginalis among others.6-10 While infection like Hepatitis C virus (HCV) is a less common STI.11
HBV is a potentially life-threatening liver infection and a major global public health problem.12-14 Sexually Transmitted Infections caused by hepatitis B virus (HBV) are associated with the risk of death arising from cirrhosis, liver, and non-liver cancers.15,16 The prevalence of HBV is highest in the World Health Organization (WHO) Western Pacific and African Regions, where 6.2% and 6.1% respectively of the adult populace is infected.13 According to the Federal Ministry of Health (FMOH) and WHO report, Nigeria has a high burden of HBV at a prevalence rate of 11.2%17 and is being classified as a hyperendemic area for HBV infection.18 The infection is usually spread from mother to child at delivery (perinatal transmission), or through horizontal transmission (exposure to infected blood), especially during the first 5 years of life from an infected to an uninfected child. It can also be spread by sexual intercourse with unvaccinated and multiple sex partners, needle stick injury, during medical, surgical and dental procedures, tattooing, piercing, and exposure to infected blood and body fluids, such as saliva, menstrual vaginal, and seminal fluids.13,19-21 Mother-to-child transmission (MTCT) of HBV is concerning because 90% of HBV-infected newborns will develop chronic infection and remain infected throughout their lives, while almost 25% of these children will die of cirrhosis, liver failure, or liver cancer later in life.19 An emerging area of opportunity to further decrease mother-to-infant transmission of HBV is the utilization of antiviral treatments during pregnancy for women at high risk of transmission.22
Hepatitis C is a liver infection caused by the Hepatitis C virus (HCV), which can lead to serious health issues, which include liver disease, liver cancer, and eventually death. Many people with HCV infection do not show any symptoms and do not know their infection.11,14 Globally, the prevalence of HCV infection varies from 1.5% to 2.3% in the most affected region while the less affected varies from 0.5% to 1.0% depending on the country.11 Nigeria has a high burden of HCV at a prevalence rate of 2.0%.17 The most common modes of transmission are exposure to small quantities of blood via injection drug use, unsafe health care, sharing needles, syringes, or other drug-injection equipment, unscreened blood transfusion, and sexual practices that result in blood exposure. HCV can as well be sexually transmitted and can be passed from an infected mother to the baby; nevertheless, these modes of transmission are less common. 11,23,24
HIV, one of the STIs caused by a virus that targets the immune system can be transmitted via the exchange of a variety of body fluids from infected people, such as breast milk, blood, semen, and vaginal secretions. HIV can also be transmitted from a mother to her child during pregnancy and delivery.25 Worldwide, the public health effect of HIV particularly as it affects women of child-bearing age cannot be emphasized enough. Nigeria has the second biggest number of individuals living with HIV and accounts for 9% of the global HIV burden.26 HIV prevalence in Nigeria has dropped from 5.1% to 3.4%27 and is currently 1.5%.28 Nigeria also reported that 4.1% of pregnant women that were attending antenatal clinics were infected with HIV.29
The venereal disease research laboratory (VDRL) test is designed to assess whether one has syphilis.30 Syphilis is an STI caused by the bacterium Treponema pallidum which infects by penetrating the lining of the mouth or genital area.9,10,30,31 Syphilis is transmitted from person to person via direct contact with a syphilitic sore, anal, vaginal, or oral sex., and from infected mothers to their unborn babies.10,31 Worldwide, syphilis is the second most common infectious cause of stillbirth.32 In 2016 the estimated global maternal syphilis prevalence is 0.69% while in Africa it is 1.52%.33 In 2017, among women attending antenatal care in Nigeria, 0.8% were affected.34
Due to shared modes of transmission, many individuals have been exposed to HBV, HCV, HIV, and syphilis infection in various combinations. A study on pregnant women attending antenatal showed that prevalence of HBV, HCV, HIV, and syphilis was 3.4%, 2.6%, 12.4%, and 0.08%, respectively while the co-infection prevalent rate was HIV-HBV (0.24%), HIV-HCV (0.14%), and HBV-HCV (0.0%).35 These co-infections have been associated with drug-related hepatotoxicity, reduced survival, drug resistance and cross-resistance, and sub-optimal response.36 Moreover, syphilis and other STIs might be indicators of ongoing behaviours and exposure that place an individual at a higher risk for acquiring HIV.10,31
Although studies have been carried out on HBV, HCV, HIV, and Syphilis prevalence among pregnant women in Ebonyi state, none has studied co-infection rate and in the rural health facilities. Therefore, the objective of this study is to assess the prevalence, co-infection, and risk factors associated with HBV, HCV, HIV and Syphilis infections among pregnant women receiving antenatal care in AMURT supported rural health facilities in Ebonyi state.
Methods
Study Design and Setting
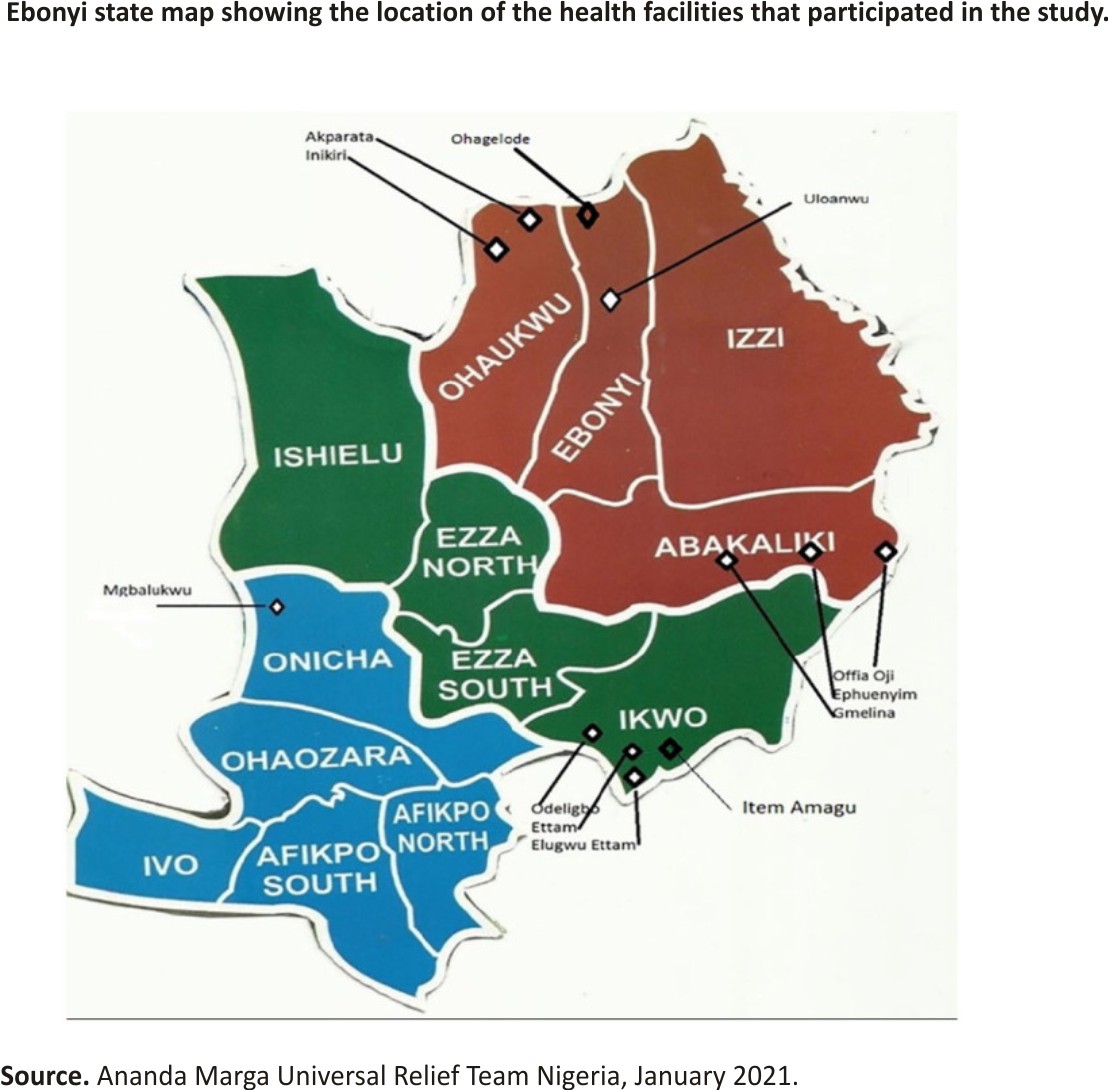
This was a retrospective study, conducted from January to December 2018 in 8 AMURT (Ananda Marga Universal Relief Team)-supported rural primary health care facilities in 5 (5) local governments out of 13 (13) in Ebonyi state southeast Nigeria (Figure 1).
Sample and Sampling Technique
All the 4657 pregnant women who attended the antenatal clinic within the study period were included in this retrospective study.
Study Instrument and Data Collection
All the antenatal records with available sociodemographic variables (mother’s age, gestational age, parity, and level of education), HIV, HBV, HCV, and syphilis screening results were used for the study. Also, risk factors such as History of Blood Transfusion and Ever has surgery as recorded were also used. However, there was no record of HBV vaccination.
Laboratory Method
Test for hepatitis B surface antigen and antibodies to HCV were determined with the Clinotech diagnostic enzyme-linked immunosorbent assay (ELISA) test kits (Clinotech Laboratories) which has sensitivity and specificity of 100% and 98.7% for hepatitis B surface antigen (HBsAg) and HCV test kits. Syphilis was checked with Venereal Disease Research Laboratory (Clinotech Biotech Inc.,) with 100% and 99.3% sensitivity and specificity respectively). Antibodies to antibodies to HIV type 1 and 2 antibodies were carried out with the use of Chembio HIV-1/2 Stat Pak (Combo Diagnostic Systems) and Unigold Determine HIV-1/2 test kit (Abbot Laboratories) with >99.9% and 98.5% specificity. All reactive samples were confirmed using ELISA and the results were reported as positive or negative. The serological tests performed in all health facilities were the same.
Statistical Analyses
The data collected were analyzed using descriptive and inferential statistics with the aid of IBM SPSS statistics version 20. Descriptive statistics were presented as frequency tables and percentages. Inferential statistical technique such as chi-square and kappa statistics (k) was used to test the hypotheses at P ≤05.
Ethics Approval and Consent to Participate
We obtained ethical approval from the Ebonyi State Ministry of Health. Informed and written consent was obtained from the Ananda Marga Universal Relief Team who released their ANC record for the study.
Results
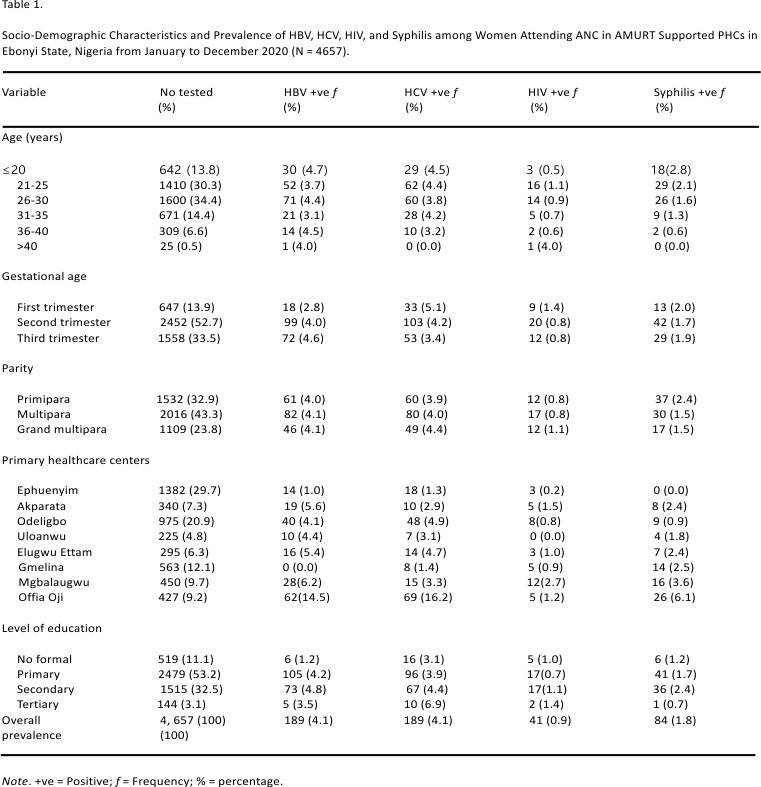
A total of 4657 pregnant women that attended antenatal care (ANC) at the 8 selected AMURT supported rural health facilities were used in the study. The socio-demographic characteristics revealed that the majority of them were 26 to 30 years (34.4%), in their second trimester (52.7%), multiparous (43.3%), and had primary education (53.2%).
Overall, 189 out of 4657 tested positive for both HBV and HCV with a prevalence rate of 4.1% respectively. Forty-one (41) out of 4657 tested positive for HIV with a prevalence rate of 0.9% while 84 out of 4657 tested positive for syphilis (VDRL test) with a prevalence rate of 1.8%.
Prevalence of HBV was more than on <20 years (4.7%), in the third trimester (4.6%), multiparous and grand multiparous (4.1%) respectively, and had secondary education (4.8%).
In addition, HCV prevalence was more among women age <20 years old (4.5%), in the first trimester gestational age (5.1%), grand multiparous (4.4%), and had tertiary education (6.9%).
The prevalence of HIV was more among women >40 years (4.0%), in the first trimester (1.4%), grand multiparous (1.1%), and had tertiary education (1.4%).
Also, syphilis prevalence was more amongst women <20 years of age 2.8%, in the first trimester (2.0%), primiparous (2.4%), and had secondary education (2.4%) (Table 1).
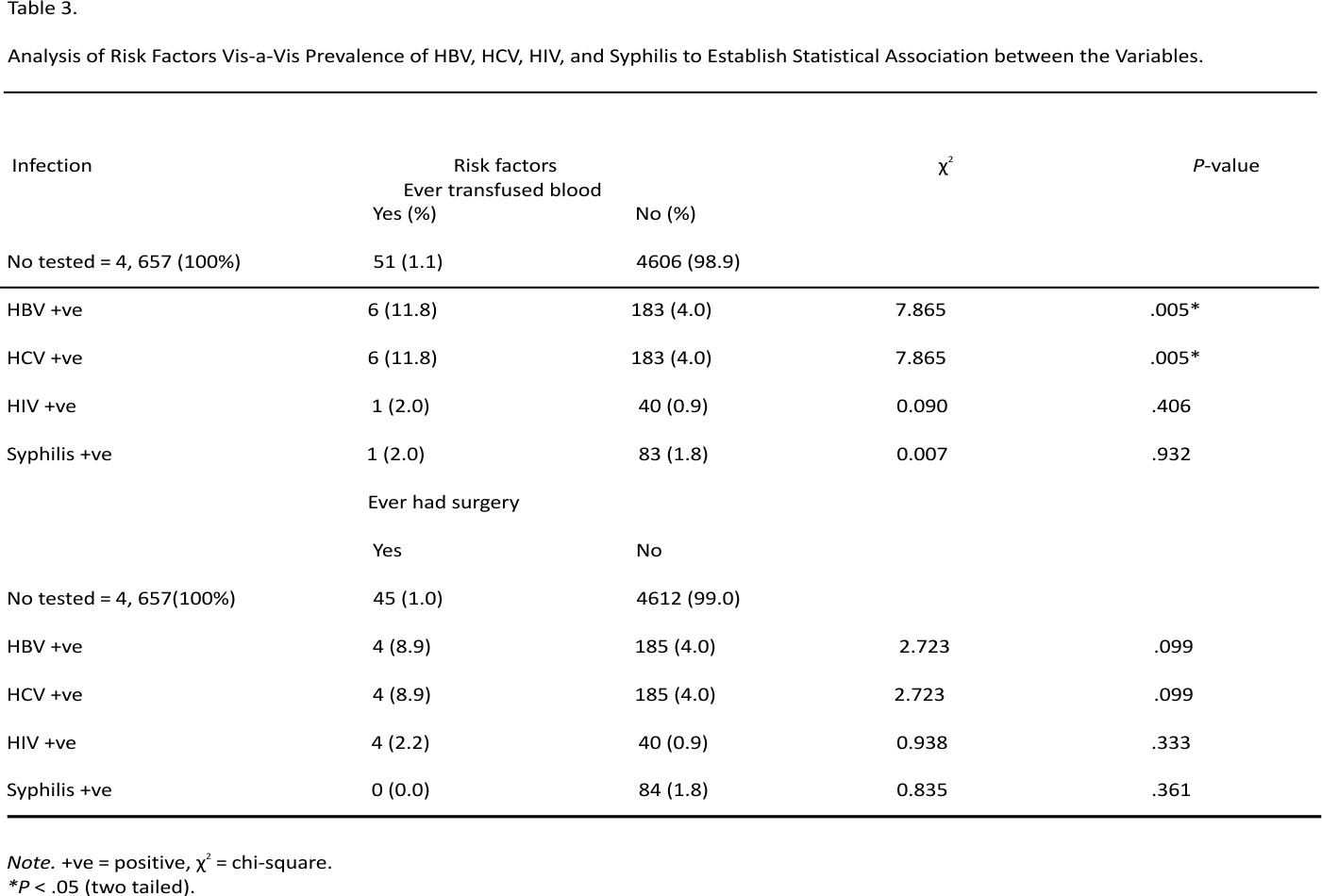
Risk factors as recorded in the ANC records were history of blood transfusion and surgery. Out of 4657 pregnant women tested, 1.1% had history of blood transfusion while 1.0% had history of surgery. Amongst those who had history of blood transfusion, 11.8% (χ2 = 7.865; P = .005*), 11.8% (χ2 = 7.865; P = .005*), 2.0% (χ2 = 0.090; P = .406) and 2.0% (χ2 = 0.007; P = .932) tested positive for HBV, HCV, HIV, and Syphilis respectively.
Furthermore, among those who had history of surgery, 8.9% (χ2 = 2.723; P = .099), 8.9% (χ2 = 2.723; P = .099), 2.2% (χ2 = 0.938; P = .333), and 0.0% (χ2 = 0.835; P = .361), tested positive for HBV, HCV, HIV, and Syphilis respectively (Table 3).
Discussion
HBV, HCV, HIV, and Syphilis infection affecting pregnant mothers may result in maternal and fetal morbidity and mortality. It could also result in chronic infection in the newborn. Prevalence and co-infection rates of HBV, HCV, HIV, and Syphilis infection varies in different parts of the world. Even in the same country, there are regional and populace precise variations.
In this study, the overall prevalence of HBV, among women attending ANC in AMURT supported PHCs in Ebonyi State, was 4.1% and is being classified as an area of medium endemicity.12 This medium prevalence was higher to the findings at different regions in Nigeria as recorded by other authors such as; 3.4% in Enugu,35 2.2% in Benin Edo state37 1.0% in Anyigba community Kogi state,38 and outside Nigeria, such as 0.4% in rural Bangladesh,39 1.6% in Egypt,40 1.02% in China41 2.0% in Guam USA,42 2.5% in Haiti (North America),43 3.07% among India women.44 The HBV prevalence recorded in this study was lower than the findings conducted in Nigeria which were: 5% in Niger Delta.,45 5.33% in Abakaliki,8 5.6% in Edo state,46 6.7% in Bauchi,47 7% in Jos,48 8.2% in Yola,49 8.5% in Kaduna,50 7.3% in Kano,51 19.8% in Keffi Nasssarawa state52 while outside Nigeria, in countries like Uganda, Southern Ethiopia, and southwest Ethiopia a prevalence of 11.8%, 4.5%, and 6.9% respectively were recorded.53-55 In comparison to the prevalence of HBV as explored in other studies, findings showed that Nigeria is an area of high endemicity, and with regard to higher hepatitis B prevalence, this infection is easily transmitted in common activities particularly in poor hygienic areas such as those found in Northern region of the where higher prevalence rates were recorded and other African regions like Ethiopia and Uganda.
Additionally, the HCV prevalence among the women in this study was 4.1%. In the Federal Ministry of Health (FMOH) and World Health Organization report, Nigeria has a high burden of hepatitis C at a prevalence rate of 2.0%,17 hence women that received antenatal health care in the AMURT supported rural health facilities in Ebonyi state have a high HCV prevalence rate. This is higher than the findings of other studies in Nigeria which were: 2.6% in Enugu,35 0.8% in Edo State,37 0.5% in Niger delta,45 1.1% in Okada, Edo state,46 0.8% among Cameroonian women,56 and 1.8% in Southern Ethiopia.54 The result is lower than the findings outside Nigeria in places like Western Ethiopia where HCV prevalence of 8.1%.57 The higher prevalence of syphilis found in this study in comparison to other countries like Cameroon and Southern Ethiopia may be caused by factors of blood transfusion which was found significant with HCV in the study.
Furthermore, the prevalence of HIV in this study was 0.9%. This is lower than the current 1.5% prevalence reported in Nigeria28 and in the studies in other regions of the country like 2.67% in Abakaliki,8 12.4% among antenatal women in Enugu35 4.1% in Niger Delta,45 7.8% reported in Minna,58 and 7.2% in Edo State.37 While the prevalence of 2.7%, 10.2%, and 9.6% was recorded outside Nigeria at Southern Ethiopia, Cameroon, and North West Ethiopia respectively.54,59,60 It is clear that there was a low HIV prevalence among the study population and this could be as a result of increased awareness and campaigns against HIV prevention, and free management of the infection that is being offered in the state.
Also, from this study, the prevalence of syphilis among antenatal attendees in Ebonyi was 1.8%. This is higher than the 0.8% prevalence in Nigeria among antenatal women, according to the World Bank collection of development indicators.34 Other reports in Nigeria revealed a prevalence of 0.08% among pregnant women at Enugu,35 0.33% in Abakaliki,8 1.7% in Cameroon,59 and 1% in Gondar, Ethiopia, among antenatal women.61 A higher prevalence of 5.0% was reported in Niger state by Buseri, Seiyaboh, and Jeremiah.45 In comparison to these findings, Ebonyi State is a syphilis endemic region. Because, after the initial infection, the Treponema pallidum (syphilis bacteria) can remain dormant in one’s body for a long time before becoming active again, mothers may not experience its signs and symptoms then thinking that they are healthy. Thus early detection and prompt treatment are important because they can be passed from mothers to unborn children.
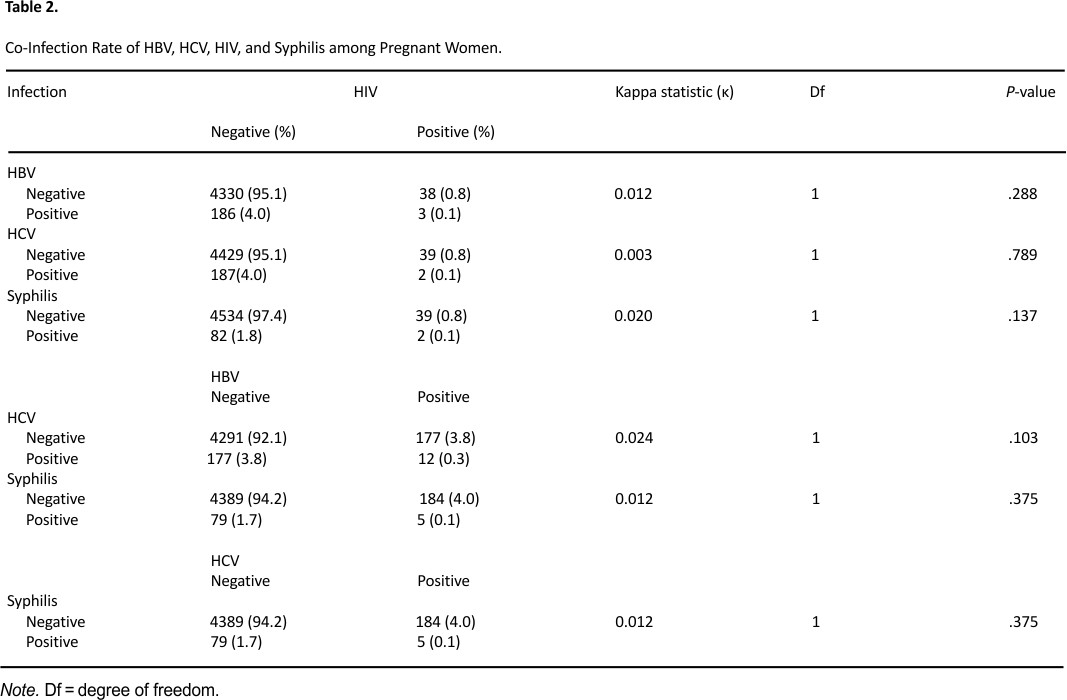
There were 3 (0.1%) cases of HBV and HIV co-infection, 2 (0.1%) cases of HCV and HIV co-infection, and 2(0.1%) case of HIV and Syphilis co-infection. This finding is lower than the HBV and HIV co-infection prevalent rate of 0.24%, 2.1% reported by other authors in Enugu and Yirgalem respectively35,62 while a higher HCV/HIV co-infection prevalence rate of 0.5% and 0.23% was reported by Ejeta and Dabsu in Ethipoia57 and Omatola et al38 in Anyigba community respectively. Additionally, there were cases 12 (0.3%) of HBV and HCV coinfection, 5 (0.1%) HBV-Syphilis co-infection and 5 (0.1%) HCV-Syphilis coinfection. These co-infections were overall 0.623% of all the study participants, though the co-infection rates recorded in this study were not statistically significant P > .05 the highest co-infected infections were HBV and HCV. This could be because Nigeria is ranked first amongst the countries of the world endemic for Schistosomiasis,63 which is a leading cause of HBV and HCV coinfection in endemic areas and pregnant women are vulnerable to it.64
On the risk factors, history of blood transfusion was significantly associated with HBV and HCV prevalence (P = .005*) but not significant with HIV (P = .090) and Syphilis (P = .932) while ever had surgery was not significant with HBV (P = .099), HCV (P = .099), HIV (P = .938), and Syphilis (P = .361) prevalence. In the same vein, EL-Shabrawi et al,40 Oladeinde et al,46 and Zenebe et al65 reported that previous history of blood transfusion and history of surgery were significantly associated with HBV infection like other studies conducted in Southern Ethiopia,54 in Arba Minch, South Ethiopia66 Addis Ababa, Ethiopia,67 and Dessie, Ethiopia.68 However, the history of blood transfusion significantly increases the risk of HIV infection in the report of Ejeta and Dabsu57 which contrasted with the findings of this present study.
The study was limited by the following: it was an already existing data for 1 year (January-December 2018) and some other risk factors could not be accessed. A more comprehensive result would have been obtained if the data was analyzed for several years, and records for previous HBV vaccination was not captured.
Conclusion
Hepatitis B and C virus, Human immunodeficiency virus, and syphilis infection are global health problems that call for high precedence in their prevention and management. The medium prevalence of HBV (4.1%) observed in this study which is much lower than the national prevalence of 11.2% reported by the Federal Ministry of Health (FMOH) and World Health Organization (WHO) should not encourage complacency because of the harmful effect of untreated maternal infection on pregnancy outcome and spread of HBV. The high burden of HCV (4.1%) in the study population is much higher than the national prevalence of 2.0% and this call for serious attention to reduce the risk of MTCT of HCV infection which will adversely affect the newborn. The low prevalence of HIV, 0.9% in this study which is higher than the national prevalence of 1.5% could be due to the increased level of awareness among antenatal women in Ebonyi and environs on the prevention of Mother to Child Transmission (PMTCT) of HIV/acquired immunodeficiency syndrome (AIDS) as well as the Health Education given to them during an antenatal visit in the health centers. The prevalence of syphilis (VDRL test) among antenatal attendees in Ebonyi is 1.8% and is higher than the 0.8% national among antenatal women. Thus, raising awareness through health education, partnerships promotion, and resource mobilization, scale-up of screening, early care and appropriate free treatment services should be provided for pregnant women attending Antenatal Care by Stakeholders to reduce the risk of spread of infections to neonates through mother to child transmission.
Acknowledgements
We acknowledge the AMURT health workers who helped pick the data of pregnant women that attended ANC in their facilities from January to December 2018.
Footnotes
Contributed by
Authors’ Contribution: MU, PS, TB, CE, DE, and OA conceptualized, designed, and coordinated the study. MU, PCIU, CAN, and RNO critically revised the manuscript. MU, IEN, NE, AU, NAA, OJC, and CAN are supported in the collection, interpretation, and data analysis. All authors read and approved the final manuscript.
Declaration of Conflicting Interests:
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
1. World Health Organization. Sexual and reproductive health. 2020. https://www.who.int/news/item/03-02-2020-the-changing-world-of-adolescent-sexual-and-reproductive-health-and-rights. Accessed July 26, 2020.
2. Ekanem EI, Ekott M, Udo AE, Efiok EE, Inyang A. Prevalence of sexually-transmitted diseases in pregnant women in Ikot-Ekpene, a rural community in Akwa Ibom State, Nigeria. OJOG. 2012;2:49-55.
3. World Health Organization. More than 1 million new curable sexually transmitted infections every day. WHO factsheet. 2019. https:// www.who.int/news-room/detail/ 06-06-2019-more-than-1-million-new-curable-sexually-transmitted-infections-every-day.
4. Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(3):217-26. doi:10.2471/ BLT.12.107623
5. World Health Organization. Sexually transmitted infections (STIs): the importance of a renewed commitment to STI prevention and control in achieving global sexual and reproductive health. 2013. https:// apps.who.int
6. Hook EW, Handsfield HH. Gonococcal infections in adults. In: Holmes KK, Sparking FP, Stamin WE, eds. Sexually-Transmitted Diseases. 4th ed. Mcgraw-Hills; 2008:627.
7. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64(RR-3):1.
8. Elom MO, Nworie A, Ugah UI, et al.. Seroprevalence of three sexually-transmitted infections (stis) among pregnant women receiving antenatal care at the federal teaching hospital, Abakaliki, Nigeria. Res J Appl Sci. 2016;12(12):1-6.
9. Center for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2017. HIV Surveillance Report; 2018;29.
10. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2018. Department of Health and Human Services; 2019.
11. World Health Organization. Hepatitis. 9 July, 2019. https://www.who.int /news-room/fact-sheets/detail/ hepatitis-c.
12. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212-2219.
13. World Health Organization, Hepatitis B. 18 July, 2019. https://www.who. int/news-room/fact-sheets/ detail /hepatitis-b.
14. The United States National Viral Hepatitis Action Plan 2017–2020. https://www.hhs.gov/hepatitis/viral-hepatitis-national-strategic-plan/index.html
15. Song C, Lv J, Liu Y, et al.. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open. 2019;2(6):e195718.
16. Soods S, Malranker S. Seroprevalence of Hepatitis B surface antigen, the antigen to the Hepatitis C virus and human immunodeficiency virus in a hospital-based population in Jaipur, Rajasthan. Indian J Community Med. 2010;35(1):165-169.10.4103 /0970-0218.62588
17. World Health Organization. Africa (/) Nigeria employs numerous strategies to create awareness on viral Hepatitis nationwide. 2020 WHO | Regional Office for Africa. https://www.afro.who.int/news/nigeria-employs-numerous-strategies-create-awareness-viral-hepatitis-nationwide
18. Ugwuja EI. Seroprevalence of hepatitis B surface antigen and liver function tests among adolescents in Abakaliki, South Eastern Nigeria. Int J Trop Med. 2010;6:1-6.
19. Atkinson W, Hamborsky J, Wolfe S, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book). 12th ed., second printing. Public Health Foundation; 2012.
20. Hu DJ, Bower WA, Ward JW. Viral hepatitis. In: Morse S, Moreland AA, Holmes KK, eds. Atlas of Sexually Transmitted Diseases and AIDS. Elsevier; 2010:203-229.
21. Eke AC, Eke UA, Okafor CI, Ezebielu IU, Ogbuagu C. Prevalence correlates and pattern of hepatitis B surface antigen in a low resource setting. Virol J. 2011;8:12.
22. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for the treatment of chronic hepatitis B. J Hepatol. 2016;63(1): 261-283.
23. Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. Am J Perinatol. 2013;30:149-159.
24. Joy JB, McCloskey RM, Nguyen T, et al.. The spread of hepatitis C virus genotype 1a in North America: a retrospective phylogenetic study. Lancet Infect Dis. 2016;16(6):698-702.
25. World Health Organization. HIV/ AIDS, 15 November, 2019. https: //www.who.int/news-room/fact-sheets /detail/hiv-aids
26. Global Health. United States Agency for International Development; 2016.
27. HIV/AIDS Information. AIDS Health Care Foundations; 2016.
28. /nigeria/prevalence-of-HIV-total-percent-of-population-ages-15-49-wb-data.html from Nigeria – Prevalence of syphilis (% of women attending antenatal care) – actual values, historical data, forecasts and projections were sourced from the World Bank (https://data. world bank. org/) in April of 2020.
29. Federal Ministry of Health. Technical Reports of National HIV Seroprevalence Sentinel Survey among Pregnant Women attending Antenatal Clinics in Nigeria; 2012.
30. Holm G, Wu B. VDRL Test, Purpose Procedure and result. Medically reviewed by Suzanne Falck, MD on November 28, 2017.
31. Centre for Disease Control and Prevention. STD Facts – Syphilis (Detailed). 2020. https://www .cdc. gov/std/syphilis/stdfact -syphilis-detailed.htm. Accessed February 20, 2020.
32. Lawn JE, Blencowe H, Waiswa P, et al.. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587-603. doi:10.1016/S0140-6736 (15) 00837-5
33. Korenromp EL, Rowley J, Alonso M, et al.. Global burden of maternal and congenital syphilis and associated adverse birth outcomes — Estimates for 2016 and progress since 2012. PLoS One. 2019; 14(2):e0211720. doi: 10.1371/journal.pone. 0211720
34. World Bank. Nigeria – Prevalence of syphilis (% of women attending antenatal care) – actual values, historical data, forecasts and projections; 2017. Sourced from the World Bank; https://data. world bank.org/.
35. Ikeako LC, Ezegwui HU, Ajah LO, Dim CC, Okeke TC. Seroprevalence of human immunodeficiency virus, hepatitis B, hepatitis C, syphilis, and co-infections among antenatal women in a tertiary institution in South East, Nigeria. Ann Med Health Sci Res. 2014; 4(6):954-958. doi:10.4103/ 2141-9248.144925 [
36. Otegbayo JA, Taiwo BO, Akingbola TS, et al.. Prevalence of hepatitis B and C seropositivity in a Nigerian cohort of HIV-infected patients. Ann Hepatol. 2008;7:152-156.
37. Oladeinde BH, Omoregie R, Oladeinde OB. Prevalence of HIV, HBV, and HCV infections among pregnant women receiving antenatal care in a traditional birth home in Benin City, Nigeria. Saudi J Health Sci. 2013;2: 113-117. http://www.saudijhealthsci. org/ text.asp?2013/2/2/113 /117916. Accessed February 20, 2020.
38. Omatola CA, Lawal C, Omosayin DO, et al.. Seroprevalence of HBV, HCV, and HIV and associated risk factors among apparently healthy pregnant women in Anyigba, Nigeria. Viral Immunol. 2019; 32(4):186-191. doi:10.1089/ vim.2018.0140
39. Shamsuzzaman M, Singhasivanon P, Kaewkungwal J, et al.. Hepatitis B among pregnant women attending health care facilities in rural Bangladesh. Southeast Asian J Trop Med Public Health. 2011; 42:1410-1413.
40. EL-Shabrawi M, Mohamed FM, EI Din Hamdi MS, Ehab M, Khamiss SS, El-Karaksy H. Prevalence of hepatitis B virus infection among Egyptian pregnant women – a single-centre study. Int J Trop Dis Health. 2013;3(2):157-168. SCIENCEDOMAIN international www.sciencedomain.org.
41. Htwe O, Coates PD, Krasu M, et al.. Prevalence of hepatitis B and other infections among pregnant women seen in a referral centre in Brunei Darussalam. Brunei Int Med J. 2013;9(4):221.
42. Abara WE, Cha S, Malik T, et al.. Hepatitis B surface antigen screening among pregnant women and care of infants of hepatitis B surface Antigen–positive mothers — Guam, 2014. MMWR. 2017: 66(19):506-508.
43. Tohmea RA, Andre-Albothb J, Tejada-Stropa A, et al.. Hepatitis B virus infection among pregnant women in Haiti: a cross-sectional serosurvey. J Clin Virol. 2016; 76: 66-71. doi:10. 1016/j.jcv.2016. 01.012
44. Khakhkhar VM, Bhura PJ, Bhuva SP, Patel CP, Cholera MS. Seroprevalence of hepatitis B amongst pregnant women attending the antenatal clinic of a tertiary care hospital, Jamnagar (Gujarat). Natl J Med Res. 2012;2:362-365.
45. Buseri F, Seiyaboh E, Jeremiah Z. Surveying infections among pregnant women in the Niger Delta, Nigeria. J Glob Infect Dis. 2010;2(3):203-211. doi:10.4103/ 0974-777X. 68525
46. Oladeinde BH, Omoregie R, Olley M, Anunibe JA. Prevalence of HIV and anaemia among pregnant women. North Am J Med Sci. 2011;3:548-551.
47. Mustapha GU, Ibrahim A, Balogun MS, Umeokonkwo CD, Mamman AI. Seroprevalence of hepatitis B virus among antenatal clinic attendees in Gamawa Local Government Area, Bauchi State, Nigeria. BMC Infect Dis. 2020; 20: 194. doi:10.1186/s12879-020-486 3-9
48. Uneke CJ, Ogbu O, Inyama PU, Anyanwie GI, Njoku MO, Idoko JH. Prevalence of hepatitis B surface antigen among blood donors and Human immunodeficiency Virus-infected patients in Jos, Nigeria. Mem Inst Oswaldo Cruz. 2005; 100(1):13-16. doi:10.1590/ S0074-02762005000100002
49. Olokoba AB, Salawu FK, Danburam A, et al.. Hepatitis B virus infection amongst pregnant women in North-eastern Nigeria-A call for action. Niger J Clin Pract. 2011; 14:10-13.
50. Omatola CA, Onoja BA, Thomas T. High rate of hepatitis B virus surface antigenemia among people living with HIV/AIDS in Kakuri, Kaduna State, North-West Nigeria. Viral Immunol. 2017;30: 516-521.
51. Yakasai IA, Ayyuba R, Abubakar IS, Ibrahim SA. Sero-prevalence of hepatitis B virus infection and its risk factors among pregnant women attending antenatal clinic at Aminu Kano Teaching Hospital, Kano, Nigeria. J Basic Clin Reprod Sci. 2012;1:49-55.
52. Mac PA, Suleiman AC, Airiohuodion PE. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care in Central Nigeria. J Infect Dis Epidemiol. 2019;5:068.10.23937 / 2474-3658/1510068
53. Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open. 2014; 4(11):e005889. doi:10.1136/bmjopen-2014-00 5889
54. Bafa TA, Egata AD. Seroepidemiological patterns and predictors of hepatitis B, C and HIV viruses among pregnant women attending antenatal care clinic of Atat Hospital, Southern Ethiopia. SAGE Open Med. 2020; 8:1-9. doi:10.1177/20503121199 00870
55. Tanga AT, Teshome MA, Hiko D, Fikru C, Jilo GK. Sero-prevalence of hepatitis B virus and associated factors among pregnant women in Gambella hospital, South Western Ethiopia: a facility-based cross-sectional study. BMC Infect Dis. 2019;19(1):602. doi:10.1186 /s128 79-019-4220-z
56. Torimiro JN, Nanfack A, Takang W, et al.. Rates of HBV, HCV, HDV and HIV type 1 among pregnant women and HIV type 1 drug resistance-associated mutations in breastfeeding women on antiretroviral therapy. BMC Pregnancy Childbirth. 2018; 18:504. doi:10.1186/s128 84-018-2120-7
57. Ejeta E, Dabsu R. Prevalence of hepatitis C virus and HIV infection among pregnant women attending antenatal care clinic in Western Ethiopia. Front Med (Lausanne). 2018;5:366. https://www.ncbi. nlm.nih.gov/pmc/articles/PMC 6352848/#B1.
58. Ndams IS, Joshua IA, Luka SA, Sadiq HO, Ayodele SB. Human immunodeficiency virus seroprevalence among pregnant women in Minna, Nigeria. Ann Niger Med. 2010; 4:14-17.
59. Dionne-Odom J, Mbah R, Rembert NJ, et al.. Hepatitis B, HIV, and syphilis seroprevalence in pregnant women and blood donors in Cameroon. Infect Dis Obstet Gynecol. 2016;2016: Article ID 4359401,8. doi:10.11 55/2016/ 4359401
60. Pineda JA, Romero-Gómez M, Díaz-García F, et al.. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779-789.
61. Mulu A, Kassu A, Tessema B, et al.. Sero-prevalence of syphilis and HIV-1 during pregnancy in a teaching hospital in northwest Ethiopia. Jpn J Infect Dis. 2007; 60:193-195.
62. Amsalu A, Ferede G, Eshetie S, Tadewos A, Assegu D. Prevalence, infectivity, and associated risk factors of hepatitis B virus among pregnant women in Yirgalem hospital, Ethiopia: implication of screening to control mother-to-child transmission. J Pregnancy. 2018;5:8435910. https://www. ncbi.nlm.nih.gov/pubmed/30174 956.
63. Oyeyemi OT. Schistosomiasis control in Nigeria: moving around the circle? Ann Glob Health. 2020; 86(1):74. doi:10.5334/aogh.2930
64. Omar HH. Impact of chronic schistosomiasis and HBV/HCV co-infection on the liver: current perspectives. Hepat Med. 2019; 11:131-136. doi:10.2147/HMER. S155962
65. Zenebe Y, Mulu W, Yimer M, Abera B. Sero-prevalence and risk factors of hepatitis B virus and human immunodeficiency virus infection among pregnant women in Bahir Dar city, Northwest Ethiopia: a cross-sectional study. BMC Infect Dis. 2014;14(1):118. doi:10.1186 /1471-2334-14-118
66. Yohanes T, Zerdo Z, Chufamo N. Seroprevalence and predictors of hepatitis B virus infection among pregnant women attending routine antenatal care in Arba Minch Hospital, South Ethiopia. Hepat Res Treat. 2016;292:90-163.
67. Desalegn Z, Wassie L, Beyene HB, Mihret A, Ebstie YA. Hepatitis B and human immunodeficiency virus co-infection among pregnant women in a resource-limited high endemic setting, Addis Ababa, Ethiopia: implications for prevention and control measures. Eur J Med Res 2016;21:16.
68. Seid M, Gelaw B, Assefa A. Sero-prevalence of HBV and HCV infections among pregnant women attending antenatal care clinic at Dessie Referral Hospital, Ethiopia. Adv Life Sci Health. 2014;1(2): 109-120.
Credits: Umoke, M., Sage, P., Bjoernsen, T., Umoke, P., Ezeugworie, C., Ejiofor, D., Agha, O., Nwalieji, C. A., Onwe, R. N., Nwafor, I. E., & Chukwu, O. J. (2021). Co-infection and Risk Factors Associated with STIs among Pregnant Women in Rural Health Facilities in Nigeria: A Retrospective Study. Inquiry: a journal of medical care organization, provision and financing, 58, 469 58021992912. https://doi.org/10.1177/0046958 021992912