Mary E. Norton, M.D.
In 1997, Dr. Yuk Ming Dennis Lo and colleagues reported that small fragments of extracellular DNA from the developing placenta are present in the maternal circulation.1 This discovery led to a revolution in the field of prenatal screening and has now been recognized by the Lasker Foundation, which has bestowed on Lo the 2022 Lasker–DeBakey Clinical Medical Research Award.
A different strategy had been pursued for years: investigators tried to extract circulating intact fetal cells, fueled by the belief that these would be accessible in a simple blood sample and could be analyzed for fetal diagnosis without the need for invasive tests such as amniocentesis. However, the identification, extraction, and accurate analysis of such cells proved to be elusive. Lo’s discovery marked a new avenue of inquiry, and over the next 14 years, others confirmed his findings, methods were developed for the analysis of cell-free DNA (cfDNA) to identify fetal trisomies, and large clinical validation studies were completed. The first clinical screening tests became available in 2011.
The intense efforts to improve screening have been spurred by the fact that trisomies, including trisomy 21 (also called Down’s syndrome), are among the most common causes of adverse pregnancy outcomes. Initially, prenatal testing for trisomies was available only through amniocentesis, which carries a risk of miscarriage, and screening was based solely on maternal age. Screening for aneuploidy (including the trisomies) improved with incremental advances involving nonspecific serum and ultrasono- graphic markers. The most effective serum-marker approach involved a combination of six serum analytes (two that were obtained in the first trimester and four in the second) and one ultrasonographic marker (measurement of nuchal translucency), each of which was measured within a narrow gestational age window. This approach had a 93% detection rate and a 5% false positive rate.2 Although the detection rate of this screening method was reasonably high, its implementation was complex, given the need for two separately obtained blood samples and an ultrasonographic examination, which were usually scheduled at different times and in different locations. Moreover, the 5% false positive rate meant that the positive predictive value was only approximately 3%. In other words, for every 20 patients who underwent screening, 1 had to decide whether to have an invasive diagnostic test and of those who received a positive result and chose to obtain diagnostic confirmation involving invasive sampling, 97% received normal results.
All this changed with the discovery of circulating fetal cfDNA, which is derived primarily from the fragmentation and release of genomic DNA from placental cells. In addition to cfDNA being released from placental cells, cfDNA is also released during the routine turnover of a person’s normal cells. Other sources of circulating cfDNA include tumors, transplanted organs, and infectious organisms. The analysis of a patient’s cfDNA can therefore provide information that is useful for the diagnosis and monitoring of cancer, the assessment of transplant-graft rejection, and the diagnosis of infectious diseases — in addition to prenatal screening for trisomies. The potential for cancer detection and monitoring was recognized in 1948 when the existence of cfDNA was first reported.3 That said, although the use of cfDNA in the context of cancer care (and in the context of transplantation) is an exciting area of research, it remains experimental, and its use in the context of infectious disease is very rare.
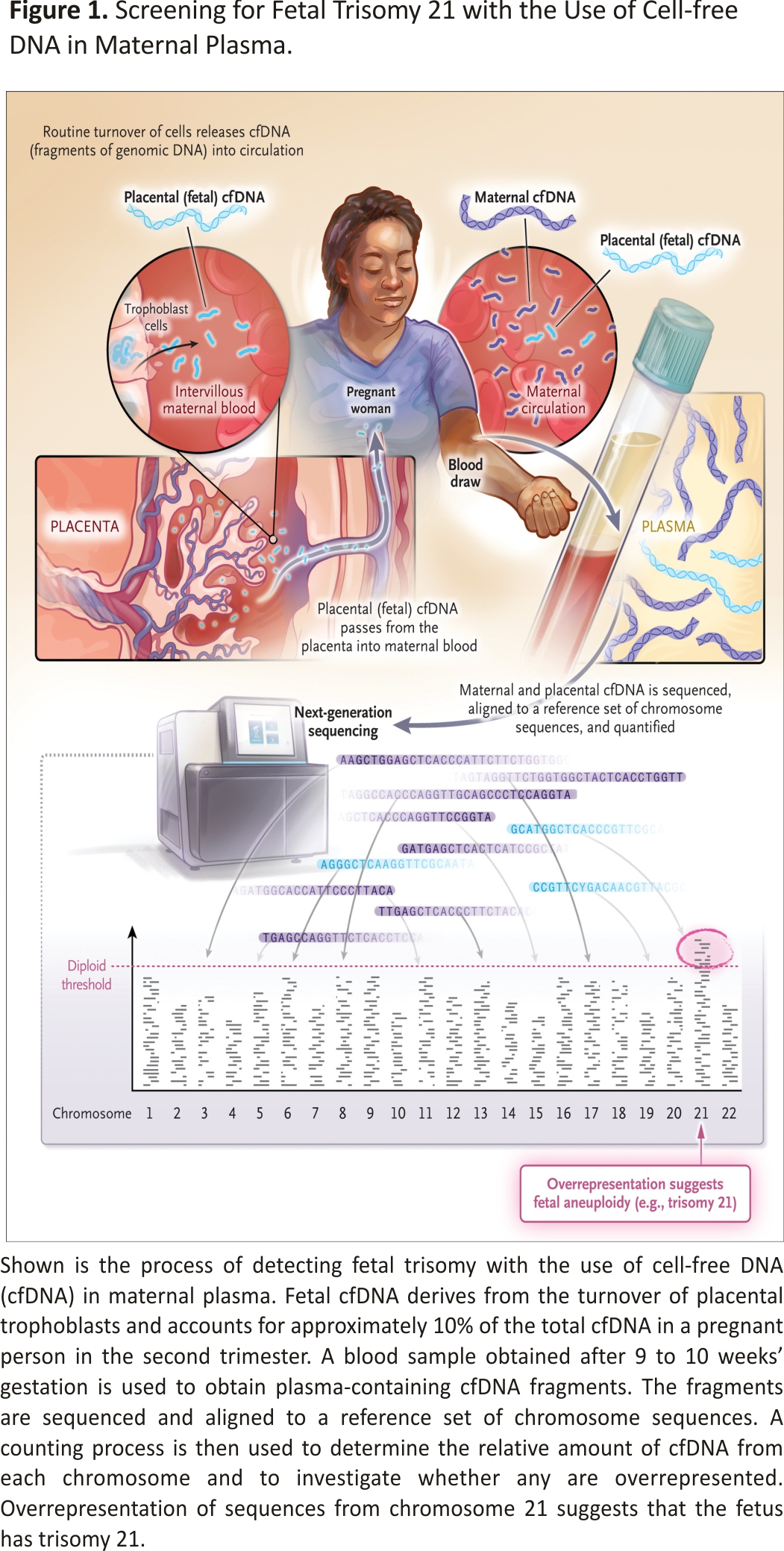
Lo’s discovery in 1997 involved the use of the polymerase chain reaction. Assessment for aneuploidies, and ultimately for other fetal genetic disorders, required far more sophisticated technologies; indeed, the widespread use of cfDNA for prenatal detection of aneuploidy was made possible only with the development of next-generation sequencing — a powerful, rapid, and inexpensive form of DNA sequencing (Figure 1).
It is possible to detect cfDNA of fetoplacental origin reliably in maternal circulation by 9 weeks gestation, and it accounts for approximately 10% of all cfDNA in maternal serum by the second trimester. Several different techniques are used and share a sensitivity for trisomy 21 detection of higher than 99%; for trisomies 18 and 13, the sensitivity is lower, although it is still above 90%.4 One of the greatest benefits of this screening method is the very high specificity of more than 99.9% for all three trisomies. Fewer than 1 in 1000 patients who undergo testing receive a false positive result for trisomy 21, and most of the positive results accurately predict an affected fetus. This is a tremendous advance over earlier screening approaches.
Prenatal cfDNA screening has been called a “disruptive innovation.”5 Certainly, it has transformed prenatal care in countries and regions where it is available. Its application to trisomy screening has been followed by further advances; cfDNA screening is now available for many microdeletion syndromes, large chromosomal deletions and duplications, and single-gene disorders. However, because these conditions are rarer, the accuracy of results is lower, with larger proportions of pregnant persons receiving false positive results than is the case for trisomy 21. A future in which the entirety of the fetal genome can be analyzed with the use of a single blood sample seems likely, even though the usefulness of such a resource in many clinical scenarios is uncertain. However, cfDNA analysis is clearly the method of choice to screen for trisomies and is an extraordinary and important product of Lo’s discovery 25 years ago.1
Author Affiliations
From the Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco.
References
1. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485-487.
2. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome. J Med Screen 1997;4:181-246.
3. Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil 1948;142:241-243.
4. Mackie FL, Hemming K, Allen S, Morris RK, Kilby MD. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG 2017;124: 32-46.
5. Warsof SL, Larion S, Abuhamad AZ. Overview of the impact of noninvasive prenatal testing on diagnostic procedures. Prenat Diagn 2015;35:972-979.
Credits: Norton ME. Circulating Cell-free DNA and Screening for Trisomies. N Engl J Med. 2022 Oct 6;387(14):1322-1324. doi: 10.1056/NEJMcibr2209405. Epub 2022 Sep 28. PMID: 36170494.