Giedre Pakuliene1, Kirilas Zimarinas 1, Irena Nedzelskiene 2, Brent Siesky 3, Loreta Kuzmiene 1, Alon Harris 3, Ingrida Januleviciene 1
Abstract
Background
Anterior chamber angle anatomy in perspective of ocular biometry may be the key element to intraocular pressure (IOP) reduction, especially in glaucoma patients. We aim to investigate anterior chamber angle and biometrical data prior to cataract surgery in patients with and without glaucoma comorbidity.
Materials and methods
This prospective comparative case-control study included 62 subjects (38 with cataract only and 24 with cataract and glaucoma). A full ophthalmic examination including, Goldmann applanation tonometry, anterior chamber swept-source optical coherence tomography (DRI OCT Triton plus (Ver.10.13)) and swept-source optical biometry (IOL Master 700 v1.7) was performed on all participants.
Results
We found that ocular biometry parameters and anterior chamber parameters were not significantly different among groups. However, when we added cut-off values for narrow angles, we found that the glaucoma group tended to have more narrow angles than the control group. IOP was higher in the glaucoma group despite all glaucoma patients having medically controlled IOP. In all subjects, anterior chamber parameters correlated well with lens position (LP), but less with relative lens position, while LP cut-off value of 5.1 mm could be used for predicting narrow anterior chamber angle parameters.
Conclusions
Cataract patients tend to develop narrow anterior chamber angles. Anterior chamber angle parameters have a positive moderate to a strong relationship with lens position. LP may be used to predict narrow angles.
Background
Cataract and glaucoma are both comorbid age-related diseases, which alter normal ocular anatomy1,2,3,4. Specifically, lens opacification induces thickening of the lens and shallowing of anterior chamber depth and these changes are even more pronounced with age 5. Several studies refer to the reduction of intraocular pressure (IOP) after phacoemulsification; however, the results vary among different authors 6,7,8,9,10. Primary angle-closure glaucoma (ACG) patients and primary angle-closure suspects (PACS) have greater IOP reduction than open-angle glaucoma (OAG) patients or otherwise healthy individuals 6,8. Even though the mechanisms behind IOP reduction after phacoemulsification are still debated, these results suggest that angle anatomy is an important landmark in IOP reduction after cataract surgery.
Varma et al. found, that almost 1 in 11 patients, referred to as “open-angle glaucoma”, was in fact found to have closed angles 11. In another study, Varma et al. found, that more than 12% of all cataract surgery referrals, referred either by an ophthalmologist or an optometrist, have closed angles or were PACS 12.
Anterior segment optical coherence tomography (AS-OCT) is a reliable anterior chamber angle evaluation method 13. The modality allows well quantifiable and highly repeatable measurements of anterior chamber angle parameters 13. Siak et al. found that even though the IOP reduction after phacoemulsification was similar between ACG and OAG groups, the AS-OCT anterior chamber angle was more open in OAG than in ACG group 14. However, they did not evaluate the lens position, which may be an important consideration.
In this analysis, we investigated AS-OCT imaging and anterior chamber angle parameters in cataract patients with or without previously diagnosed OAG in perspective of ocular biometry and lens position results. The focus of our study included cataract surgery patients, referred by an ophthalmologist, with no suspicion of closed or narrow anterior chamber angle. To the best of our knowledge, this is the first study evaluating AS-OCT anterior chamber angle parameters in cataract patients with or without OAG in perspective of biometry results and lens position preoperatively.
Methods
This prospective comparative case-control study was carried out in the Lithuanian University of Health Sciences, Ophthalmology Department in 2018– 2019. All procedures were approved by the Kaunas Regional Biomedical Research Ethics Committee and all subjects signed informed consent prior to participation.
Inclusion criteria: patients, who were scheduled for cataract surgery and having an open-angle (Shaffer III-IV gonioscopically), as referred by district ophthalmologist. The study group consisted of cataract patients over 18 years with diagnosed and medically controlled OAG, while the control group consisted of cataract patients over 18 years with no other eye disease.
Exclusion criteria: subjects with vision < 6/24 (Snellen chart), glaucoma suspects, patients with obvious lens subluxation or lens swelling (to exclude lens-induced glaucoma), suspected angle closure a priori. We excluded OAG patients with IOP > 21 mmHg, and additionally, medications for IOP reduction were recorded for the OAG patients.
All of the subjects received a full ophthalmic examination, including IOP via Goldmann applanation tonometry, AS-OCT (DRI OCT Triton plus (Ver.10.13)) for anterior chamber angle tomograms (measuring angle opening distance at 500 μm from scleral spur (AOD500), trabecular iris space at 500 μm and 750 μm from scleral spur (TISA500 and TISA750) and swept-source optical biometry (IOL Master 700 v1.7) for ocular biometry (axial length (AL), anterior chamber depth (ACD), lens thickness (LT), central corneal thickness (CCT), spherical equivalence (SE), white-to-white corneal diameter (WTW). ACD was measured from the epithelium. IOP was measured after AS-OCT and biometry, to avoid artefacts. AS-OCT imaging was performed under darkroom conditions without pupil dilation and AS-OCT images were processed and evaluated using Fiji program package15. The anterior chamber angles were manually evaluated by two independent observers (G.P. and K.Z.) in a blinded manner. The intraobserver repeatability and interobserver agreement were excellent (Intraclass correlation coefficient (ICC) ≥0.9). All of the AS-OCT scans were performed using the “Line 6 mm” option with external fixation. AS-OCT scans were performed at the 3,6,9,12 o’clock position of anterior angle structures. We chose to proceed with 3 and 9 o’clock positions, because had a lot of scleral artefacts, and not all tomograms were appropriate for evaluation. This could be due to the age of the patients.
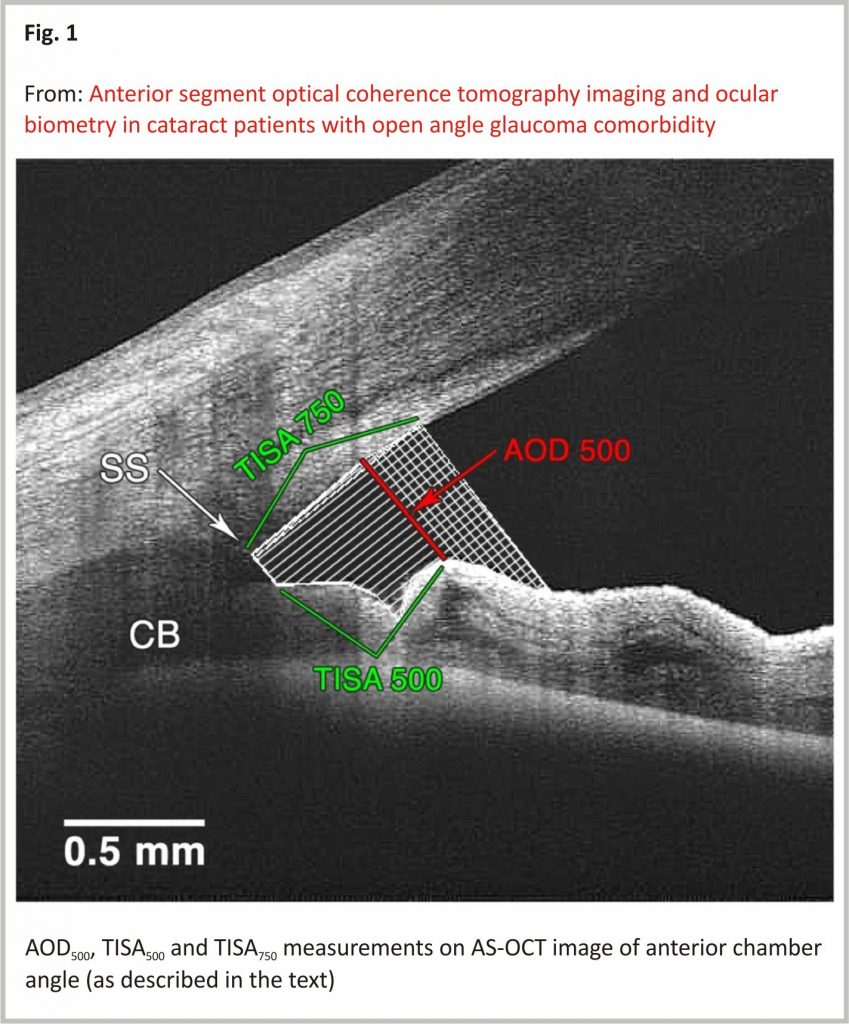
The measurements were made as follows (Fig. 1):
1. Angle opening distance (AOD500) – the distance from the point on the cornea (which was 500 μm from the scleral spur) to a perpendicular point on the iris (as described by Pavlin et al.16). Angle opening distance at 750 μm (AOD750) was used in TISA750 measurement.
2. Trabecular iris space area (TISA500 and TISA750)17 – the area with defining boundaries of:
1. Anterior wall – AOD500 or AOD750 respectively;
2. Posterior wall – a line, beginning at the scleral spur, drawn perpendicularly from the inner scleral wall to the iris;
3. Superior wall – corneoscleral surface between anterior and posterior walls;
4. Inferior wall – iris surface between anterior and posterior walls.
The TISA500 and TISA750 were measured as described by Radhakrishnan et al. 17.
Narrow anterior chamber angle is considered < 20° and < 10° upon gonioscopy according to Shaffer classification, with probable and possible angle-closure respectively 18. Individuals with anterior chamber angle < 20° were considered PACS, despite not having any glaucomatous changes 18. According to Radhakrishnan et al., the cut-off value for indicating occludable angles (≤10°) in AS-OCT was 191μm for AOD500, 0.11mm2 for TISA500 and 0.17mm2 for TISA750 17. We chose the same cut-off values to reevaluate the data. There were several studies, which suggested different cut-off values for occludable anterior chamber angles in AS-OCT 17,19,20,21. We chose previously mentioned cut-off values because we found similar results in our pilot study (mean and median).
Swept-source optical biometry was performed in lightroom conditions without pupil dilation. The measurements collected were:
1. Axial length (AL) (mm);
2. Anterior chamber depth (ACD) (mm);
3. Lens thickness (LT) (mm);
4. Spherical equivalent (SE) (D);
5.Horizontal corneal diameter (white to white WTW) (mm);
6. Central corneal thickness (CCT) (μm).
LP and RLP were derivative values from AL, ACD and LT. LP was found adding ACD and ½ LT. RLP was found LP dividing by AL 22.
In order to compare AOD500, the calculated sample size to provide 80% power to detect a difference of 50 μm with SD of 58 μm 17 between control and study patients was at least 22 in each group assuming two-sided tests and a 95% significance level.
Primary and secondary outcomes
The main outcomes of our study included comparing anterior chamber angle and ocular biometrical measurements between cataract patients with or without OAG. The secondary outcomes were the assignation to whether the anterior chamber angle was open or narrow and finding correlations between anterior chamber angle parameters and lens position (LP) and relative lens position (RLP).
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 23.0. (Armonk, NY: IBM Corp) program package. An appropriate statistical test was chosen to evaluate the results. Kolmogorov-Smirnov test was used to determine the distribution of the data. The student’s t-test was used for normally distributed independent samples. Quantitative data were presented as Mean (SD). Mann-Whitney U test was used for non-parametric independent samples; the data was presented as Median (IQR). Pearson’s Correlation Coefficient (PCC) was used for correlations. In order to assess minimally false-negative and minimally false-positive results with the greatest accuracy, the method of ROC (Receiver Operating Characteristics) curve was used. Statistical significance was set at p < 0.05.
Results
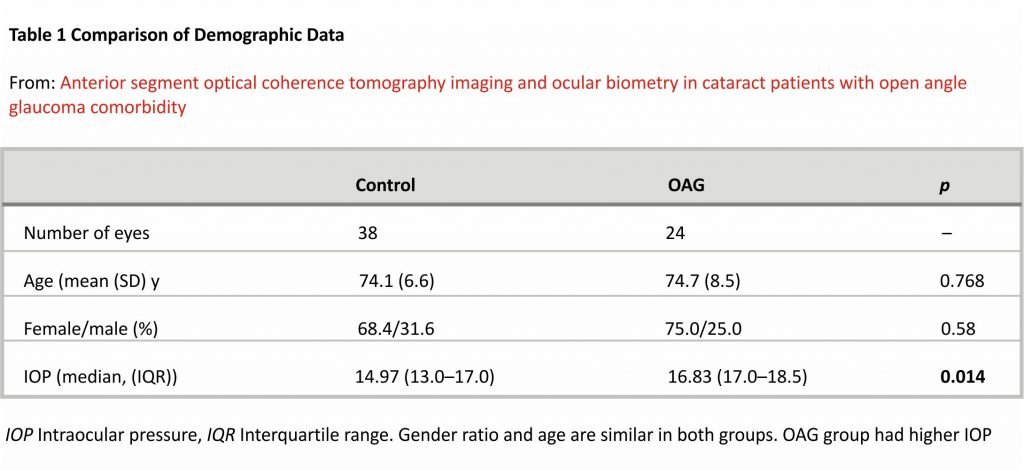
Sixty-two subjects were included in the study with a control group of 38 (61.3%) subjects and the glaucoma group included 24 (38.7%) subjects. All of the subjects were of Caucasian ethnicity. Gender ratio and age were similar in both groups (Table 1). Even though OAG group subjects had medically controlled glaucoma, their IOP was statistically significantly higher than the control group.
Ocular biometry
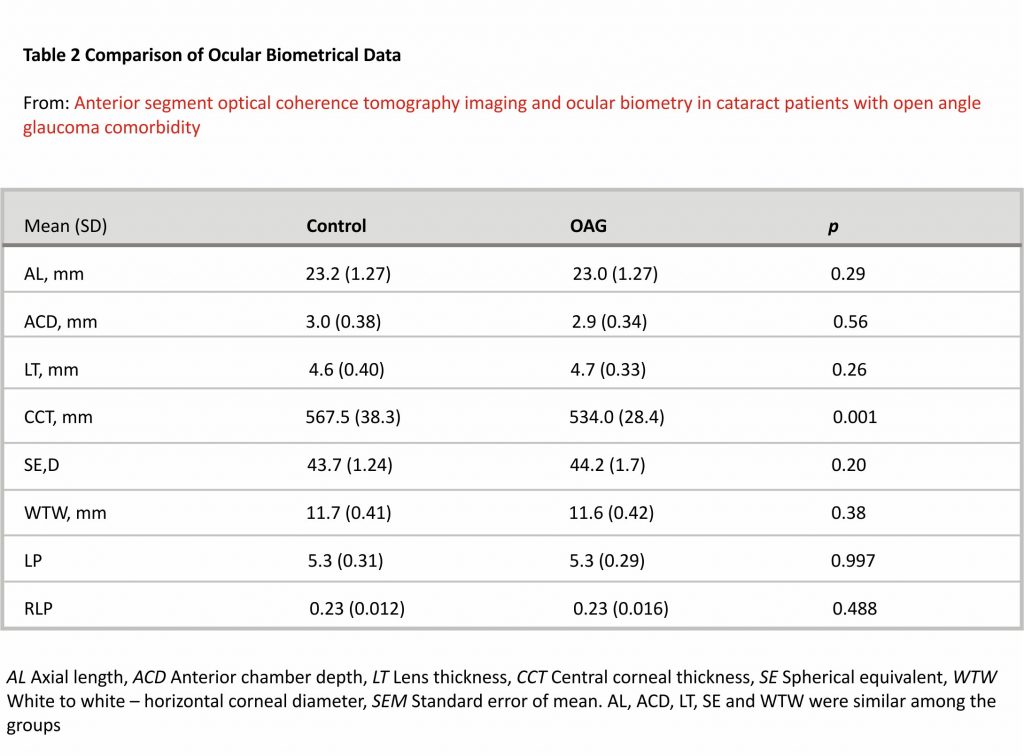
Ocular biometry measurements were similar in both groups, except for CCT, which was statistically significantly lower in the OAG group (Table 2).
We found a moderate negative correlation between ACD and LT in both controls (r = − 0.595, p < 0.001) and glaucoma groups (r = − 0.521, p = 0.009). In the control group, ACD showed a moderate positive correlation with AL (r = 0.559, p < 0.001), however, no correlation between ACD and AL was found in the glaucoma group (p = 0.318) (PCC).
A moderate negative correlation was seen between AL and SE in both the control group (r = − 0.448, p = 0.005) and glaucoma groups (r = − 0.463, p = 0.026). CCT did not correlate with AL, ACD or LT in neither of the groups (p > 0.05) (PCC).
Anterior chamber angle
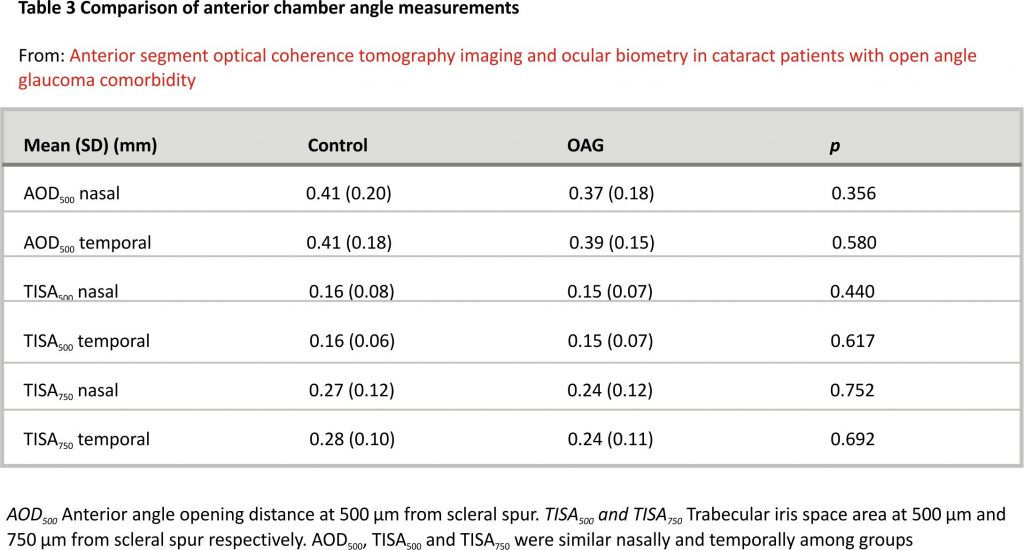
Anterior chamber angle measurements were similar between the two groups (Table 3).
We chose the previously mentioned cut-off values to reevaluate the data 17. The percentage of narrow angles in cataract and cataract with OAG groups according to AOD500 were approximately 11.1 and 21.7% (p > 0.05), according to TISA500 were 25.0 and 30.4% (p > 0.05), according to TISA750 were 22.2 and 30.4% (p > 0.05). The percentages of narrow angles were similar in both groups nasally and temporally, with a tendency of a slightly higher percentage in the OAG group.
The number of different hypotensive substance used by OAG patients did not correlate with anterior chamber angle parameters, nor ACD (p > 0.05, PCC).
LP, RLP and AOD500
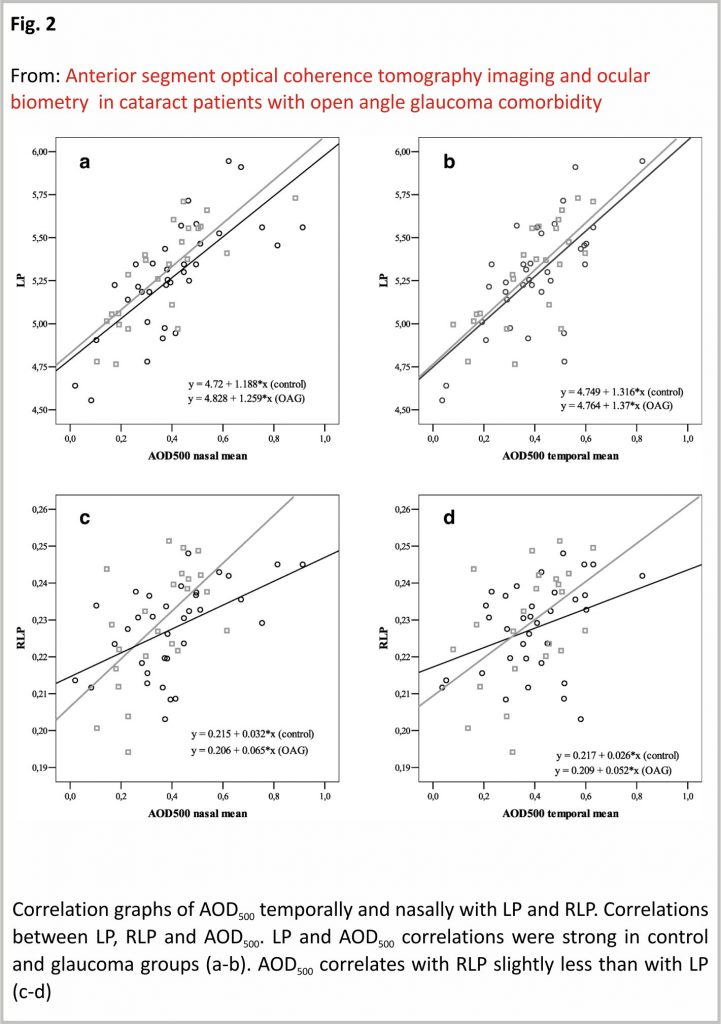
LP showed a strong positive correlation with AOD500 nasally (r = 0.733, p < 0.001) and temporally (r = 0.690, p < 0.001) in the control group and accordingly nasally (r = 0.777, p < 0.001) and temporally (r = 0.727, p < 0.001) in OAG group. RLP showed a moderate positive correlation with AOD500 nasally (r = 0.524, p = 0.001) and a weak positive correlation temporally (r = 0.362, p = 0.034) in the control group. In the OAG group, RLP showed a moderate positive correlation with AOD500 nasally (r = 0.587, p = 0.036), and temporally (r = 0.493, p = 0.017) (Fig. 2).
LP, RLP and TISA500
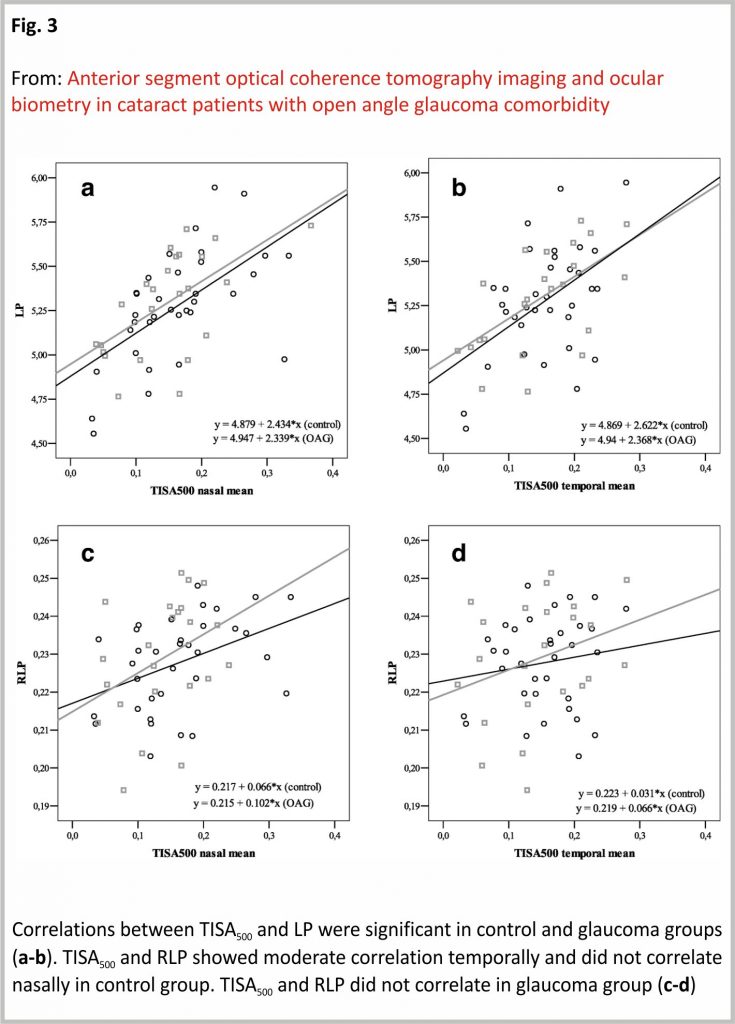
LP showed a moderate positive correlation with TISA500 nasally (r = 0.593, p < 0.001), and temporally (r = 0.489, p = 0.003) in the control group while strong positive correlation nasally (r = 0.597, p = 0.002) and moderate positive correlation temporally (r = 0.591, p = 0.003) in OAG group. RLP showed a moderate positive correlation with TISA500 nasally (r = 0.420, p = 0.013), however did not show any correlation with TISA500 temporally (p = 0.412) in the control group. RLP did not show any correlation with TISA500 neither nasally, nor temporally in the OAG group (p > 0.05) (PCC) (Fig. 3).
LP, RLP and TISA750
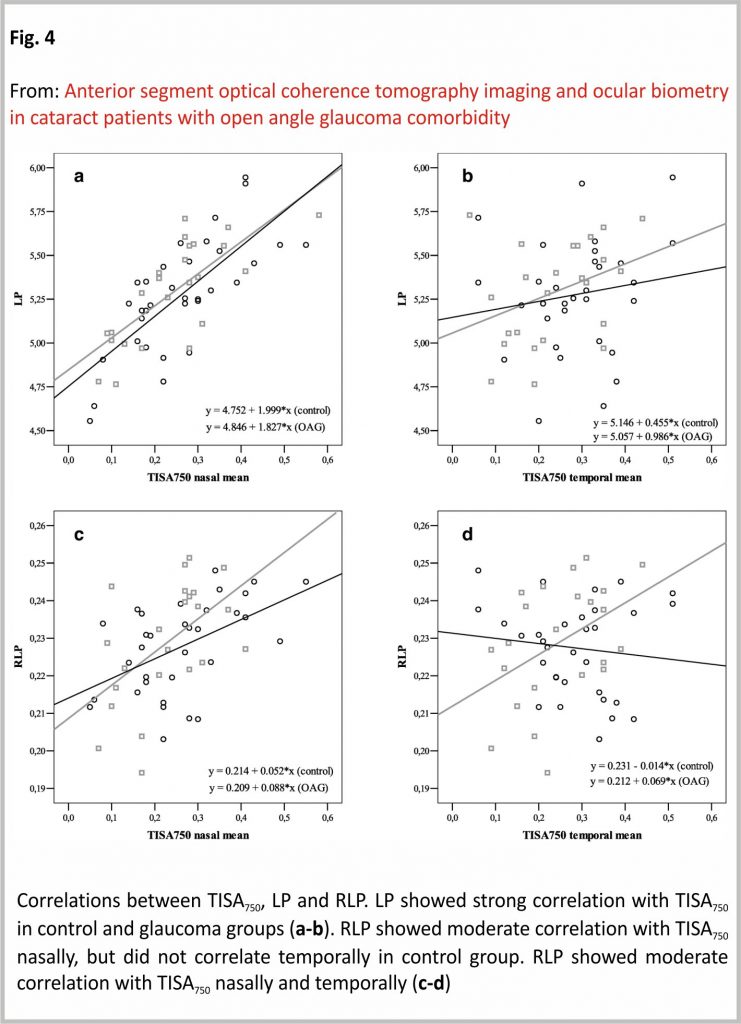
LP showed a strong positive correlation with TISA750 nasally (r = 0.738, p < 0.001), but not temporally (p > 0.05) in the control group. In the glaucoma group, LP showed a strong positive correlation with TISA750 nasally (r = 0.747, p < 0.001), but no correlation temporally (p > 005). RLP showed a moderate positive correlation with TISA750 nasally (r = 0.506, p = 0.003), however, did not correlate with the temporal side (p > 0.05) in the control group. RLP showed a moderate positive correlation with TISA750 nasally (r = 0.536, p = 0.008) and temporally (r = 0.436, p = 0.048) in the OAG group (PCC) (Fig. 4).
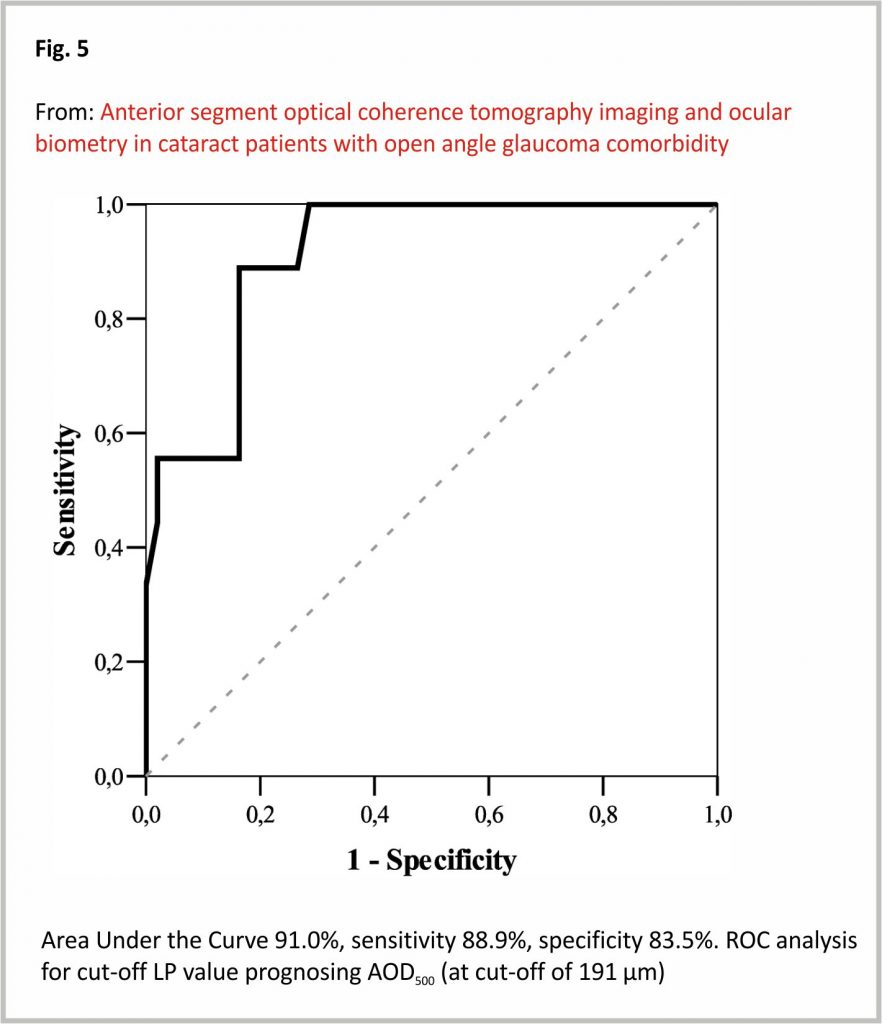
The LP and anterior chamber angle parameters were not statistically different among groups, so we proceeded following calculation combining both groups. ROC analysis the cut-off value of LP 5.1 (mm), considering AOD500 cut-off value (191 μm)17. If LP was > 5.1 (mm), AOD500 was < 191 μm in 2.4% of cases (n = 1). If LP was < 5.1 mm, AOD500 was < 191 μm in 50% of cases (n = 8) (p < 0.001) (Fig. 5).
Discussion
Our study aimed to evaluate anterior chamber angle parameters and biometrical ocular data in individuals with cataract and with or without OAG. We did not find any statistically significant differences between ocular biometry parameters in control and OAG groups (regarding AL, ACD, LT, SE, WTW and the derivate values LP and RLP). The OAG group had slightly narrower anterior chamber angles than the control group, however, the difference was statistically insignificant.
The CCT was lower in the OAG group and this result was in agreement with previous studies 23. We found that mean LT was 4.6 mm in the control group and 4.7 mm in the OAG group which were similar to results previously reported in cataract patients without glaucoma in studies by Shammas et al. (mean LT 4.6 mm) 5, Jivrajka et al. (mean LT 4.93 mm) 24 and Hoffer et al. (mean LT 4.63 mm) 25. Shammas et al. also found that the most increase of LT attributed to anterior cortex space 5.
Wang et al. found that non-glaucomatous white individuals had more posteriorly positioned lenses, than Asians, African Americans and Hispanics 26. However, in our study we found, that LP preoperatively was more anteriorly positioned in both groups than in the white individuals’ sample in Wang et al. study 26. The difference could occur due to a slightly older sample in our study. The LP and RLP in our study were similar in control and OAG groups. According to our results, cataract patients with and without OAG showed no differences in LP prior to cataract surgery. Importantly, we found the cut-off LP value of 5.1 mm was predictive of whether the patient had a narrow-angle. In the future, this measurement could be used to predict narrow angles with ocular biometry, alongside gonioscopy.
Mean AOD500, TISA500 and TISA750 were statistically similar in control and OAG groups. This suggests that individuals, who developed cataract, had in general similar anterior chamber angle parameters, despite having or not having glaucoma. Using the same cut-off values as Radhakrishnan et al., we also found parameters were slightly different between cataract vs. cataract and glaucoma groups17. Although the differences between groups were not statistically significant, we found that the OAG group tended to have narrow angles slightly more often than the control group. In addition to that, we found, that IOP was statistically significantly higher in OAG than in the control group, despite the fact that OAG patients had sufficiently medically controlled IOP.
Our study showed a slightly higher percentage of narrow angles in both control and OAG groups than Varma et al. 12. They found that 12.9% of cataract referrals had PACS/angle closure, however, it is important to note that our study patients were much older than in Varma et al. study 12. This difference in age could explain a higher percentage of PACS/angle closure. Additionally, Varma et al. used gonioscopy to determine narrow angles, while we used AS-OCT; and the different evaluation method could lead to different results 12. Varma et al. suggested that this percentage of undiagnosed narrow angles in their study could be because gonioscopy was underperformed and narrow angles were missed 12. We suggest that since the anterior chamber is a dynamic structure, in some cases the overall view may alter over time and differ from previous examinations, especially in cataract cases. However, the Varma et al. study did not specify whether glaucoma had been already diagnosed in study group 12. The undetected narrow angles for individuals with glaucoma may have an impact on IOP management.
The literature suggests that LT has an inverse relationship with AL and ACD 27. In our analysis, we found that anterior chamber angle parameters (AOD500, TISA500) had a strong positive correlation with LP (which was calculated using ACD and LT). We also found that anterior chamber angle parameters depended on ACD and LT (LP) more than ACD, LT and AL (RLP). This could be explained by an increase in lens thickness during cataract formation in mainly anterior cortical space, which influenced anterior chamber angle parameters and due to this AL influence was less significant 5.
Radhakrishnan et al. in AOD500, TISA500 and TISA750, showed slightly larger parameters measuring temporal quadrant comparing to nasal quadrant. This was neither emphasized as a statistically significant difference, nor of overall importance in the original article 17. Our measurements between nasal and temporal quadrants were slightly, yet not statistically different. When we computed correlations between angle measurements and RLP, we noticed that in the control group correlations were weaker or absent in the temporal quadrant, but remained at least moderate in the nasal quadrant. In the glaucoma group, AOD500 and TISA750 maintained a strong or moderate correlation with RLP in both quadrants, but TISA500 lost it. This may be partially explained by the lens tilt, as previous studies suggest lens tilt to be up to 5 degrees with outward nasal orientation with mirror symmetry in both eyes 28, 29.
We also found, that if LP were below the cut-off value (< 5.1), it was more likely, that AOD500 was < 191 μm, which falls into a “narrow-angle” category.
Our study had several advantages as compared to similar studies. We used objective evaluation of ocular biometric parameters, which were more accurate and were possible even through dense nucleus 30,31,32. Another advantage was, that we used AS-OCT anterior chamber angle measurements inaccurate closeup images, which did not require contact and our measurements were highly repeatable.
Along with advantages our study also had several limitations to acknowledge. First, our study did not differentiate, which part of the lens was most affected by the cataract – nuclear, subcapsular or cortical, as did Shammas et al. 5. Instead, we provided objective measurement (LP and RLP), which change, if the lens thickens in the cortex, nucleus, subcapsular masses, or in a combination with lens parts. We also did not differentiate the OAG group by glaucoma medications (only by a number of different substances), which may influence results. Additionally, it is worth to mention, that all of the measurements, presented in our study, were derivative and not direct, yet remaining objective.
Conclusion
Ocular biometry data and anterior chamber angles were similar among cataract patients with and without glaucoma. CCT was lower in glaucomatous subjects. Patients with cataract, despite having or not having glaucoma, tended to develop narrow angles. Patients with cataract and glaucoma had higher preoperative IOP, compared to the control group, even though they had medically controlled glaucoma. AS-OCT helped to obtain useful quantifiable information on anterior chamber angle anatomy. LP cut-off value of 5.1 mm was found to be able to differentiate between open and narrow-angle (AOD500) with high sensitivity and specificity. IOL-Master700 was an effective tool to evaluate ocular biometry parameters even through a dense nucleus. These approaches could lay a new perspective to future studies and future studies with different age groups and different stages of glaucoma are needed to evaluate the possible influence on IOP change due to narrowing of angles during cataract formation. In addition, LP as a predictor of narrow anterior chamber angle may be important to evaluate in larger longitudinal studies.
Availability of data and materials
The data used in this study is available at reasonable request to the corresponding author.
Abbreviations
AS-OCT: Anterior segment optical coherence tomography
ACG: Angle-closure glaucoma
ACD: Anterior chamber depth
AL: Axial length
AOD500 : Angle opening distance
CCT: Central corneal thickness
IOP: Intraocular pressure
LT: Lens thickness
LP: Lens position
OAG: Open-angle glaucoma
PACS: Primary angle-closure suspects
RLP: Relative lens position
SE: Spherical equivalent
TISA500 and TISA750 : Trabecular iris space area 500μm and 750μm from scleral spur respectively
WTW: Horizontal corneal diameter (white to white)
References
1. Ling JD, Bell NP. Role of cataract surgery in the Management of Glaucoma. Int Ophthalmol Clin. 2018;58:87–100.
2. Satou T, Shimizu K, Tsunehiro S, et al. Relationship between crystalline Lens thickness and shape and the identification of anterior ocular segment parameters for predicting the intraocular Lens position after cataract surgery. Biomed Res Int. 2019;2019:9.
3. Grimfors M, Mollazadegan K, Lundström M, et al. Ocular comorbidity and self-assessed visual function after cataract surgery. J Cataract Refract Surg. 2014;40:1163–9.
4. Gaspar R, Pinto LA, Sousa DC. Corneal properties and glaucoma: a review of the literature and meta-analysis. Arq Bras Oftalmol. 2017;80:202–6.
5. Shammas HJ, Shammas MC. Measuring the cataractous lens. J Cart Refract Surg. 2015;41:1875 –9.
6. Baek SU, Kwon S, Park IW, et al. Effect of phacoemulsification on intraocular pressure in healthy subjects and glaucoma patients. J Korean Med Sci. 2019;34: 1294–307.
7. Coh P, Moghimi S, Chen RI, et al. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in glaucomatous versus nonglaucomatous eyes. Investig Ophthalmol Vis Sci. 2016; 57:2593–9.
8. Chen PP, Lin SC, Junk AK, et al. The effect of phacoemulsification on intraocular pressure in Glaucoma patients: a report by the American Academy of ophthalmology. Ophthalmology. 2015;122:1294 – 307.
9. Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. Randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36:407–12.
10. Bilak S, Simsek A, Capkin M, et al. Biometric and intraocular pressure change after cataract surgery. Optom Vis Sci. 2015;92:464–70.
11. Varma DK, Simpson SM, Rai AS, et al. Undetected angle closure in patients with a diagnosis of open-angle glaucoma. Can J Ophthalmol. 2017;52:373–8.
12. Varma DK, Kletke SN, Rai AS, et al. Proportion of undetected narrow angles or angle closure in cataract surgery referrals. Can J Ophthalmol. 2017;52:366–72.
13. Campbell P, Redmond T, Agarwal R, et al. Repeatability and comparison of clinical techniques for anterior chamber angle assessment. Ophthalmic Physiol Opt. 2015;35: 170–8.
14. Siak J, Quek D, Nongpiur ME, et al. Anterior chamber angle and intraocular pressure changes after phacoemulsification: a comparison between eyes with closed-angle and open-angle glaucoma. J Glaucoma. 2016;25:e259–64.
15. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9: 676–82.
16. Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–9.
17. Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy in measuring anterior chamber angle. Arch Ophthalmol. 2005;123: 1053 –9.
18. Foster PJ, Aung T, Nolan WP, et al. Defining ‘occludable’ angles in population surveys: drainage angle width, peripheral anterior synechiae, and glaucomatous optic neuropathy in east Asian people. Br J Ophthalmol. 2004;88:486–90.
19. Cheung CY Lui, Liu S, #, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Investig Ophthalmol Vis Sci 2010; 51: 4040–4046.
20. Wirbelauer C, Karandish A, Häberle H, et al. Noncontact goniometry with optical coherence tomography. Arch Ophthalmol. 2005;123:179–8 5.
21. Grewal DS, Brar GS, Jain R, et al. Comparison of Scheimpflug imaging and spectral-domain anterior segment optical coherence tomography for detection of narrow anterior chamber angles. Eye. 2011;25:603–11.
22. Hsu CH, Kakigi CL, Lin SC, et al. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in nonglaucomatous patients with open angles. Investig Ophthalmol Vis Sci. 2015;56:7807 – 13.
23. Sng CCA, Ang M, Barton K. Central corneal thickness in glaucoma. Curr Opin Ophthalmol. 2017;28: 120–6.
24. Jivrajka R, Shammas MC, Boenzi T, et al. Variability of axial length, anterior chamber depth, and lens thickness in the cataractous eye. J Cataract Refract Surg. 2008;34: 289 –94.
25. Hoffer KJ. Axial Dimension of the Human Cataractous Lens. Arch Ophthalmol. 1993. Epub ahead of print;111. https://doi.org/10.10 01/archopht.1993.010900700 32014.
26. Wang D, Amoozgar B, Porco T, et al. Ethnic differences in lens parameters measured by ocular biometry in a cataract surgery population. PLoS One. 2017;12: 1– 11.
27. Shammas JH. A-scan biometry of 1000 cataractous eyes. In: Ossoinig KC, editor. Ophthalmic echography. Documenta Ophthalmologica proceedings series; 1987. p. 57–6 3.
28. Hirnschall N, Buehren T, Bajramovic F, et al. Prediction of postoperative intraocular lens tilt using swept-source optical coherence tomography. J Cataract Refract Surg. 2017;43: 732–6.
29. Wang L, Guimaraes de Souza R, Weikert MP, et al. Evaluation of crystalline lens and intraocular lens tilt using a swept-source optical coherence tomography biometer. J Cataract Refract Surg. 2019;45: 35–40.
30. Akman A, Asena L, Güngör SG. Evaluation and comparison of the new swept-source OCT-based IOLMaster 700 with the IOLMaster 500. Br J Ophthalmol. 2016;100: 1201–5.
31. Bullimore MA, Slade S, Yoo P, et al. An evaluation of the IOLMaster 700. Eye Contact Lens Sci Clin Pract. 2019;45:117–23.
32. Shajari M, Cremonese C, Petermann K, et al. Comparison of axial length, corneal curvature, and anterior chamber depth measurements of 2 recently introduced devices to a known biometer. Am J Ophthalmol. 2017;178:58–64.
Acknowledgements
Prof. Alon Harris would like to disclose that he receives remuneration from AdOM for serving as a consultant and a board member; he serves on the board of Phileas Pharma and has received reimbursement from Thea for a speaking engagement. Prof. Harris also holds an ownership interest in AdOM, Luseed, Oxymap, and QuLent.
We thank Justinas Ryskus for preparing the illustrations.
Funding
We received no external funding.
Author information
Affiliations
Ophthalmology Department, Lithuanian University of Health Sciences, Eiveniu g. 2, 50161, Kaunas, Lithuania
Giedre Pakuliene, Kirilas Zimarinas, Loreta Kuzmiene & Ingrida Januleviciene
Biostatistician, Odontology Faculty, Department of Dental and Oral Pathology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Irena Nedzelskiene
Icahn School of Medicine at Mount Sinai, New York, NY, USA
Brent Siesky & Alon Harris
Contributions
IJ and AH contributed to the idea and design of the study. GP carried out the research, LK supervised the carry-out and findings of the study. IN was responsible for carrying out statistical analysis. GP and KZ performed the measurements and calculations of the findings. GP wrote the draft manuscript. LK, BS, IJ, and AH edited the manuscript. All of the authors revised the final version of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Giedre Pakuliene.
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Lithuanian Bioethics Committee (No. BE-2-52) and adhered to the tenets of the Declaration of Helsinki. Each participant signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Credits: Pakuliene, G., Zimarinas, K., Nedzelskiene, I. et al. Anterior segment optical coherence tomography imaging and ocular biometry in cataract patients with open-angle glaucoma comorbidity. BMC Ophthalmol 21, 127 (2021). https:// doi.org/10.1186/s12886-021-01874-x