Shuen-Ru Yang MD, MS1, Mu-Hung Tsai MD2,Chung-Jye Hung MD3, Shu-Ling Peng Md4 ,Nan-Tsing Chiu MD5, Yu-Hui Huang MS6, Hui-Jen Tsai MD, PhD1,7,8
- 1Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Department of Radiation Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Department of Pathology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Department of Nuclear Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 6Nursing Department of National Cheng Kung University Hospital, Tainan, Taiwan
- 7National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan
- 8Division of Hematology and Oncology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Abstract
Objective
Anaplastic thyroid cancer (ATC) is a rare thyroid cancer subtype with a devastating prognosis. Novel treatment strategies are under investigation to improve the survival of patients with ATC.
Methods
We present a case of recurrent ATC treated with a combination of radiation therapy (RT) and pembrolizumab, a programmed death-1 inhibitor, with a durable complete response.
Results
A 63-year-old woman underwent total thyroidectomy and left neck lymph node dissection and was diagnosed with papillary carcinoma in December 2017. She received radioiodine in April 2018. However, a left neck mass was noted in April 2018 with biopsy demonstrating ATC with 95% positivity for programmed death-ligand 1 immunostaining. Positron emission tomography showed fluorodeoxyglucose uptake in the left thyroid bed and multiple lymph nodes in the left retropharyngeal, left neck, and right upper paratracheal areas. Hypofractionated RT for the recurrent areas was initiated in August 2018, and concomitant pembrolizumab was given 2 days after RT. A total of 10 cycles of pembrolizumab (2 mg/kg) were given every 3 weeks. The computed tomography scan after completion of RT and 3 cycles of pembrolizumab showed shrinkage of the neck lymph nodes. The serial follow-up computed tomography scans showed further shrinkage of the lymph nodes, and there was no recurrence of ATC as of October 2020.
Conclusion
We describe an ATC case successfully treated with a combination of RT and pembrolizumab with a durable response of 26 months and acceptable toxicities. This result warrants further investigation of this combination regimen in the treatment of ATC.
Keywords
anaplastic thyroid cancer, an immune checkpoint inhibitor, pembrolizumab, radiation therapy
Abbreviations
ATC, anaplastic thyroid cancer; CI, confidence interval; CT, computed tomography; NSCLC, non–small-cell lung cancer; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death-ligand 1, RT, radiation therapy; RR – response rate
Introduction
Anaplastic thyroid cancer (ATC) is a rare subtype of thyroid cancer with a devastating prognosis. Treatment strategies for ATC include surgical resection, radiation therapy (RT), chemotherapy, or combinations of these treatment modalities.1 The prognosis of ATC is very poor, with a median overall survival (OS) of 3.16 months according to an analysis of data from the Surveillance, Epidemiology, and End Results database from 1986 to 2015.2 Many factors, such as metastasis, age, socioeconomic status, and treatment strategies (surgery and radiation), may influence the survival of patients with ATC.2 Several novel treatment strategies for ATC have been under investigation. Here, we report a case of metastatic ATC successfully treated with a combination of RT and pembrolizumab, a programmed death (PD)-1 inhibitor, with a durable response of >2 years.
Case Report
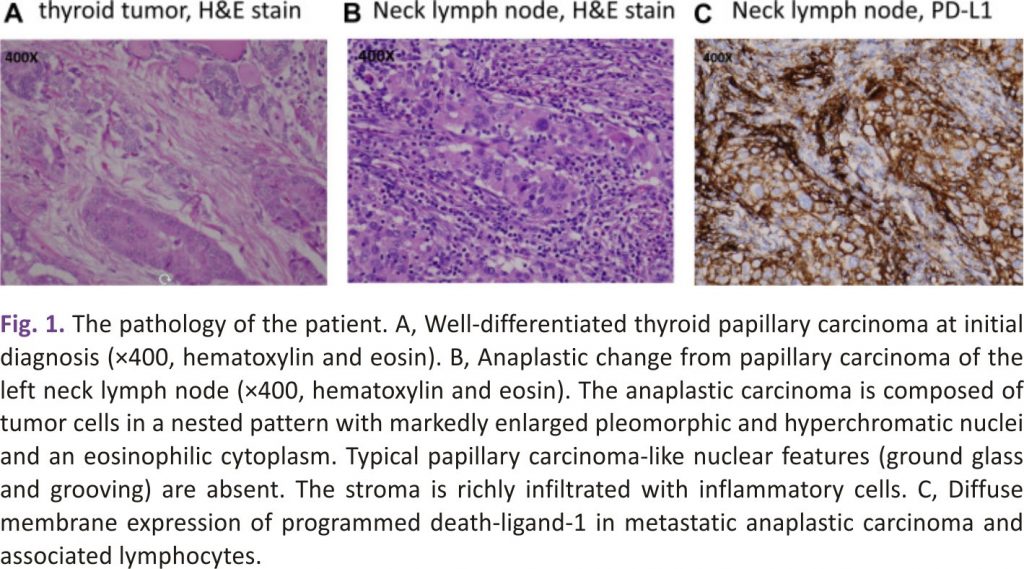
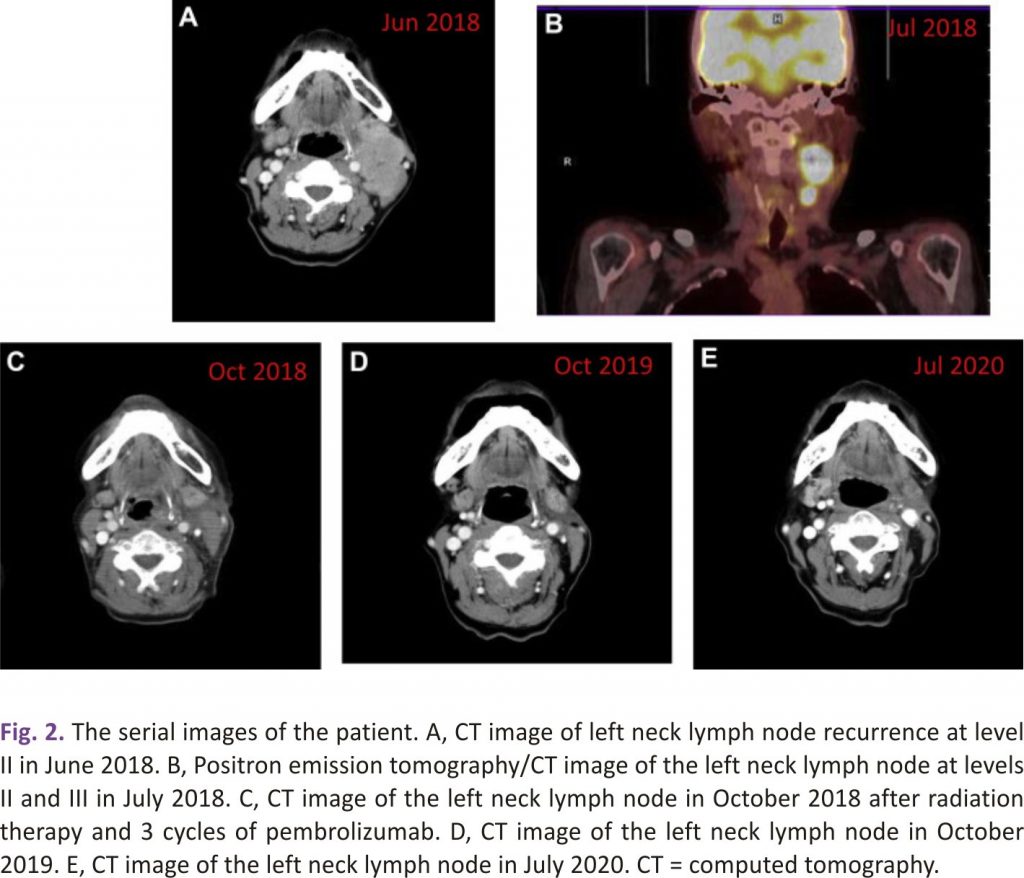
A 63-year-old woman was diagnosed with thyroid cancer. She underwent total thyroidectomy, central neck dissection, and left lateral neck dissection with removal of lymph nodes at level III and level IV in December 2017. She underwent an excisional lymph node biopsy of the left neck 3 weeks later. The pathology results of the left thyroid tumour and lymph nodes showed papillary carcinoma of the classical type (Fig. 1A) with a pathologic stage of T2N1M0 (left thyroid tumour, 2.5 × 1.5 × 1 cm; 13 metastatic lymph nodes of 18 removed lymph nodes in the first surgery and 2 metastatic lymph nodes of 2 removed lymph nodes in the second surgery). Her thyrotropin, thyroglobulin, and antithyroglobulin antibody levels were 0.07 μIU/mL, <0.2 ng/mL, and negative (negative: <115 IU/mL), respectively, in January 2018. She received subsequent radioiodine therapy with 110 mCi of iodine-131 in April 2018. A whole-body iodine-131 scan after iodine-131 treatment showed hot spots in the anterior neck, which were suspected to be remnant thyroid tissue or lymph nodes. However, a left neck mass was noted in April 2018. A contrast-enhanced computed tomography (CT) scan in June 2018 showed enlarged and conglomerated enhancing masses in the left lateral neck levels II and III, up to 2.4 cm. The lesions were suspected to be metastatic lymphadenopathy. Surgical removal of the left neck masses was not performed because the surgeon considered it too difficult for complete resection. Incisional biopsy of the left neck lymph node at level II was performed and demonstrated metastatic anaplastic carcinoma with high (95%) PD-ligand 1 (PD-L1) expression (Fig 1 B and 1 C). A positron emission tomography /CT scan was performed and showed a recurrent tumour in the left thyroid bed and metastatic lymphadenopathy in the left retropharyngeal, left neck, and right upper paratracheal areas (Fig. 2). The staging of the recurrent tumour was T3N1bM0, stage IVB. The thyrotropin, thyroglobulin, and antithyroglobulin antibody levels were 0.03 μIU/mL, <0.2 ng/mL, and negative, respectively, in May 2018. She received RT with a prescription of 5000 cGy in 25 fractions to the left thyroid bed, left neck, and retropharyngeal lymph node region and 7500 cGy in 25 fractions to the left neck and right upper paratracheal gross lymphadenopathy using a simultaneous integrated boost technique combined with pembrolizumab (2 mg/kg) every 3 weeks, beginning in August 2018. The first dose of pembrolizumab was given 2 days after the start of RT. A significant decrease in the size of the left neck lymphadenopathy was noted by a CT scan in October 2018 after completion of RT and 3 cycles of pembrolizumab. She received a total of 10 cycles of pembrolizumab until April 2019. There was no recurrence of neck lymph-adenopathy as of October 2020. Serial CT images of the patient are shown in Fig. 2. The adverse events included grade 1 radiation dermatitis, dysphagia, xerostomia, and anorexia, likely attributed to RT. Grade 1 hepatitis and adrenal insufficiency developed after pembrolizumab was administered and were managed adequately.
Discussion
Many patients with ATC (20%-90%) have coexisting well-differentiated carcinoma or a history of previously resected differentiated or poorly differentiated carcinoma.1 Our case is such a situation. However, the outcome of ATC is poor irrespective of coexisting or previously resected differentiated carcinoma. A multimodal approach, including surgery, RT, and systemic therapy, is recommended for ATC, particularly stages IVA and IVB.1 For stage IVC cases, a clinical trial or palliative care is suggested.1 Chemotherapy with a taxane, anthracycline, platinum, or a combination of these agents is a commonly used systemic therapy for ATCs.1 The efficacy of chemotherapy in ATCs is limited.1 Lenvatinib, a multitargeted kinase inhibitor, was shown to achieve a response rate (RR) of 24% and a median OS of 7.4 months in 17 ATC cases in a Japanese phase II study.3
BRAFV600E mutations are found in 20% to 50% of ATCs.4 The prognostic role of BRAF mutations in ATC is unclear. The combination of dabrafenib (a BRAF inhibitor) and trametinib (a mitogen-activated protein kinase inhibitor) has been shown to achieve an overall RR of 69% and a 1-year progression-free survival rate of 79% in patients with ATC with BRAFV600E mutations.4
The prognosis of ATC has been improved by novel treatments in recent years. Maniakas et al 5 analyzed the OS of patients with ATC at MD Anderson Cancer Center from 2000 to 2019 and divided them into 3 groups according to the date of presentation (2000-2013, 2014-2016, and 2017-2019). The median OS of all 3 cohorts combined was 9.5 months. The median OS significantly improved in the third cohort (2017-2019) compared with that of the first cohort (2000-2013), with a hazard ratio of 0.5 (95% confidence interval [CI], 0.38-0.67). Targeted therapy, the addition of immunotherapy to targeted therapy, and surgery following neoadjuvant BRAF-directed therapy were factors associated with improved survival.5
Immune checkpoint inhibitors targeting PD-1 or PD-L1/2, alone or in combination with chemotherapy, have been shown to be efficacious in multiple cancer types, particularly in tumours or microenvironments with high PD-L1 expression.6 High proportions (approximately 90%) of ATCs are positive for PD-L1.7 Kollipara et al8 reported an ATC case successfully treated with vemurafenib and nivolumab with durable remission of 20 months. That case was diagnosed as papillary carcinoma, and the patient underwent thyroidectomy, lymph node dissection, and iodine-131 treatment. The patient had neck lymph node recurrence, and the pathology demonstrated ATC and positivity for the BRAFV600E mutation and PD-L1 staining. Vemurafenib and nivolumab were given after treatment failure with 2 cycles of chemotherapy with doxorubicin and cisplatin.8 Cabanillas et al9 reported an ATC case successfully treated with a combination of multiple treatment modalities. That case had a thyroid mass of 8 cm encasing the left common carotid artery with tracheal deviation and significant lymphadenopathy and was diagnosed as ATC by fine-needle aspiration. The patient was treated with dabrafenib and trametinib with a partial response after failure with weekly paclitaxel and subsequent carboplatin plus nab-paclitaxel but still developed a slightly larger left supraclavicular lymph node, which was also confirmed to be ATC. The patient had multiple mutations detected from cell-free DNA and lymph nodes, including BRAFV600E, TP53R175H, EGFRG322S, and NRASQ61K, and BRAF amplification. Pembrolizumab was given, and a decrease in lymph node size was noted. The patient then underwent surgery, and papillary carcinoma was noted in the right lymph node in addition to ATC in the primary tumour and left lymph nodes. The patient then received dabrafenib plus pembrolizumab, followed by 6 weeks of RT plus chemotherapy (cisplatin plus pembrolizumab), followed by triple therapy with dabrafenib plus trametinib plus pembrolizumab. The patient was reported to have an excellent quality of life approximately 11 months after diagnosis.9 The case demonstrated a combination of BRAF- and immune-directed therapy as a neoadjuvant and postsurgery treatment for advanced ATC. In a retrospective study from MD Anderson Cancer Center, 12 patients with advanced ATC were treated with tyrosine kinase inhibitors with the subsequent addition of salvage pembrolizumab. The best overall RR was 42%, and the median progression-free survival and OS with the addition of pembrolizumab were 2.96 (95% CI, 2.2-3.7) and 6.93 (95% CI, 1.7-12.15) months, respectively.10 Spartalizumab, an anti-PD-1 monoclonal antibody, has been reported to achieve an RR of 29% and a 1-year survival rate of 52% in PD-L1-positive ATCs. The RR was even higher (35%) when the PD-L1 level of the tumours was ≥50%.7
RT is commonly used to treat various cancers for local or palliative control of tumours. A hypofractionated dose (fraction size >2 Gy) has the potential to increase the RR to tumours that respond poorly to traditional RT.11,12 In addition, a low dose or hypofractionated dose of RT can modulate tumour cells in several ways to make tumour cells more susceptible to immune cell reactivity.13 The combination of RT with immunotherapy has been shown to generate synergistic effects for cancers in preclinical models and selected patients by modulating immunity in the microenvironment.13,14 In a secondary analysis of the KEYNOTE-001 phase I trial, the clinical activity and toxicity of pembrolizumab were evaluated for patients with advanced non–small-cell lung cancer (NSCLC). Patients with NSCLC who had received prior RT had a longer median progression-free survival than those without prior RT (4.4 [95% CI, 2.1-8.6] months vs 2.1 [1.6-2.3] months, P = .025).15 In a randomized phase III study, durvalumab, a PD-L1 inhibitor, or placebo was used as consolidation therapy for stage III NSCLC patients who did not have disease progression after 2 or more cycles of platinum-based chemoradiotherapy. The patients who received durvalumab had a longer median progression-free survival than those who received the placebo 16.8 [95% CI, 13.0-18.1] months vs 5.6 [95% CI, 4.6-7.8] months (P < .001).16. These studies demonstrated the synergistic effect of combining RT or chemoradiotherapy with immunotherapy in lung cancer. A phase 2 study using a combination of pembrolizumab and chemoradiotherapy as initial treatment for ATC was conducted. Three patients were enrolled. Although a favourable locoregional tumour response was noted initially, the study was halted after all patients died within 6 months due to progression in 1 case and treatment complications with pneumonitis and pneumonia in the other 2 cases.17 The toxicities of combining immunotherapy with chemoradiotherapy for ATC should be carefully considered after the results of the phase 2 trial, although this combination is expected to be effective for ATC. We did not add chemotherapy in our case, and only mild toxicity developed, which suggests the tolerability of this combination in ATC. In our case, pembrolizumab was given 2 days after initiation of RT, which may modulate tumour cells and the microenvironment before pembrolizumab acts. However, whether the sequence of immunotherapy and RT affect the antitumor response needs further investigation.
Conclusion
We have demonstrated an ATC case treated with a combination of RT and pembrolizumab with a durable response of 26 months accompanied by only mild toxicities. This result suggests the potential of combining RT and immune checkpoint inhibitors to treat ATCs, and further investigation is warranted.
Acknowledgement
We thank Jeffrey S. Chang for the English editing of this paper. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors have no multiplicity of interest to disclose.
References
1. R.C. Smallridge, K.B. Ain, S.L. Asa, et al. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer Thyroid, 22 (11) (2012), pp. 1104-1139
CrossRefView Record in ScopusGoogle Scholar
2. B. Lin, H. Ma, M. Ma, et al. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Transl Res, 11 (9) (2019), pp. 5888-5896
View Record in ScopusGoogle Scholar
3. S. Takahashi, N. Kiyota, T. Yamazaki, et al. A phase II study of safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol, 15 (7) (2019), pp. 717-726
CrossRefView Record in ScopusGoogle Scholar
4. V. Subbiah, R.J. Kreitman, Z.A. Wainberg, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer J Clin Oncol, 36 (1) (2018), pp. 7-13
View Record in ScopusGoogle Scholar
5. A. Maniakas, R. Dadu, N.L. Busaidy, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol, 6 (9) (2020), pp. 1397-1404
CrossRefView Record in ScopusGoogle Scholar
6. S.P. Patel, R. Kurzrock. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther, 14 (4) (2015), pp. 847-856
View Record in ScopusGoogle Scholar
7. J. Capdevila, L.J. Wirth, T. Ernst, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol, 38 (23) (2020), pp. 2620-2627
CrossRefView Record in ScopusGoogle Scholar
8. R. Kollipara, B. Schneider, M. Radovich, S. Babu, P.J. Kiel. Exceptional response with immunotherapy in a patient with anaplastic thyroid cancer. Oncologist, 22 (10) (2017), pp. 1149-1151
CrossRefView Record in ScopusGoogle Scholar
9. M.E. Cabanillas, R. Ferrarotto, A.S. Garden, et al. Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid, 28 (7) (2018), pp. 945-951
View Record in ScopusGoogle Scholar
10. P.C. Iyer, R. Dadu, M. Gule-Monroe, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid cancer. J Immunother Cancer, 6 (1) (2018), p. 68
View Record in ScopusGoogle Scholar
11. A. Oweida, A. Phan, B. Vancourt, et al. Hypofractionated radiotherapy is superior to conventional fractionation in an orthotopic model of anaplastic thyroid cancer. Thyroid, 28 (6) (2018), pp. 739-747
View Record in ScopusGoogle Scholar
12. N. Takahashi, H. Matsushita, R. Umezawa, et al. Hypofractionated radiotherapy for anaplastic thyroid carcinoma: 15 years of experience in a single institution. Eur Thyroid J, 8 (1) (2019), pp. 24-30
CrossRefView Record in ScopusGoogle Scholar
13. A. Kumari, S.S. Simon, T.D. Moody, C. Garnett-Benson. Immunomodulatory effects of radiation: what is next for cancer therapy. Future Oncol, 12 (2) (2016), pp. 239-256
CrossRefView Record in ScopusGoogle Scholar
14. E. Chajon, J. Castelli, H. Marsiglia, R. De Crevoisier. The synergistic effect of radiotherapy and immunotherapy: a promising but not simple partnership. Crit Rev Oncol Hematol, 111 (2017), pp. 124-132. Article Download PDFView Record in ScopusGoogle Scholar
15. N. Shaverdian, A.E. Lisberg, K. Bornazyan, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol, 18 (7) (2017), pp. 895-903. Article Download PDFView Record in ScopusGoogle Scholar
16. S.J. Antonia, A. Villegas, D. Daniel, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med, 377 (20) (2017), pp. 1919-1929.
CrossRefView Record in ScopusGoogle Scholar
17. A.V. Chintakuntlawar, J. Yin, R.L. Foote, et al. A phase 2 study of pembrolizumab combined with chemoradiotherapy as initial treatment for anaplastic thyroid cancer. Thyroid, 29 (11) (2019), pp. 1615-1622
CrossRefView Record in ScopusGoogle Scholar
Credits: Shuen-Ru Yang, Mu-Hung Tsai, Chung-Jye Hung, Shu-Ling Peng, Nan-Tsing Chiu, Yu-Hui Huang, Hui-Jen Tsai, Anaplastic Thyroid Cancer Successfully Treated With Radiation and Immunotherapy: A Case Report, AACE Clinical Case Reports, 2021, ISSN 2376-0605, https://doi.org/10.1016/j.aace.2021.03.003.
(https://www.sciencedirect.com/science/article /pii/S2376060521000432)