Frank R. Lin, M.D., Ph.D
AFFILIATIONS
From the Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore.
Dr. Lin can be contacted at flin1@jhmi.edu or the Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins School of Medicine, JHOC 6210, 601 N. Caroline St., Baltimore, MD 21287.
A 72-year-old man presents for a routine visit accompanied by his wife. He has no health issues, but his wife volunteers that she has concerns about his hearing. On further questioning, the patient notes problems with hearing and understanding others but attributes these issues to his wife and other family members not speaking clearly. The patient’s wife notes that she has heard about the recent availability of over-the-counter hearing aids as well as media reports that hearing loss is linked with the risk of dementia. She wonders whether her husband could benefit from using over-the-counter hearing aids. How would you respond?
The Clinical Problem
Age-related declines in hearing gradually affect every person during life. A person’s ability to hear depends on the inner ear (cochlea) precisely encoding sounds into neural signals, which are then processed and decoded into meaning at the cortical level. Pathologic processes that occur at any level of this pathway from the ear to the brain can adversely affect hearing, but age-related hearing loss involving the cochlea is the most common cause.1
KEY CLINICAL POINTS
AGE-RELATED HEARING LOSS
• Age-related declines in hearing gradually and progressively affect every person during life, initially manifesting as difficulty understanding speech in background noise or other specific situations.
• Age-related hearing loss detrimentally affects communication and social functioning and is considered to be one of the most clinically significant risk factors for cognitive decline and dementia.
• Management of age-related hearing loss is focused on the use of communication strategies and technologies (hearing aids and cochlear implants) to increase the clarity of the speech signal.
• Evidence from a randomized trial suggests that hearing aid use can improve communication and quality of life and may reduce cognitive loss within 3 years in older adults who are at risk for cognitive decline.
• Technology and regulatory changes now enable adults to self-test and track their hearing using a smartphone (www.hearing number.org) and to purchase over-the-counter hearing aids. This approach aligns with broader trends toward empowering consumers with knowledge and options to act on their health without a clinician intermediary.
Age-related hearing loss is characterized by the progressive loss of the sensory hair cells of the inner ear, which are responsible for encoding sound into neural signals.1,2 Unlike other cells throughout the body, sensory hair cells in the inner ear cannot regenerate, and these cells are progressively lost for life owing to the cumulative effects of multiple etiologic processes. The strongest risk factors for age-related hearing loss include older age, lighter skin colour as an indicator of cochlear pigmentation (given that melanin is protective in the cochlea), male sex, and noise exposure.3 Other risk factors include cardiovascular disease risk factors such as diabetes, smoking, and hypertension, which can contribute to microvascular injury to cochlear blood vessels.3
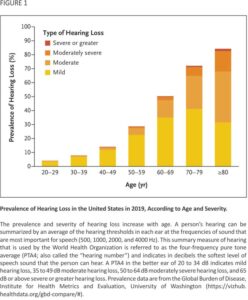
Beginning in early adulthood, hearing diminishes gradually, particularly about sounds at higher frequencies. The prevalence of clinically significant hearing loss increases across the life span, nearly doubling with every decade of life such that more than two-thirds of all adults 60 years of age or older have some form of clinically significant hearing loss (Figure 1).3,4 In the United States in 2019, approximately 72.9 million, or one in five persons were estimated to have hearing loss.4
Epidemiologic studies have shown associations between hearing loss and impaired communication, cognitive decline,5 dementia,6 higher medical costs,7 and other adverse health outcomes.3,8 Research over the past decade has particularly focused on the effects of hearing loss on cognitive decline and dementia, and based on this evidence, the Lancet Commission on Dementia concluded in 2020 that hearing loss in middle and late life was the single largest potentially modifiable risk factor for dementia, accounting for 8% of all dementia cases.6 The main mechanisms through which hearing loss has been hypothesized to increase the risks of cognitive decline and dementia include adverse effects of hearing loss and impoverished auditory encoding of sound on cognitive load, brain atrophy, and social isolation.9,10
Strategies and Evidence
CLINICAL PRESENTATION
Age-related hearing loss manifests gradually and subtly over time in both ears without any clear inciting event. It affects the audibility and clarity of sounds and a person’s everyday communication experience. Persons with mild hearing loss are often not aware of diminishing hearing and instead perceive their hearing difficulties as being attributable to external reasons (e.g., others not speaking clearly and background noise). At greater levels of hearing loss, persons may increasingly notice trouble with speech clarity even in quiet settings and can find conversations in noisier settings exhausting, given the increased cognitive effort that is required for processing the degraded speech signal.11 Often, family members are most aware of patients’ hearing difficulties.
EVALUATION
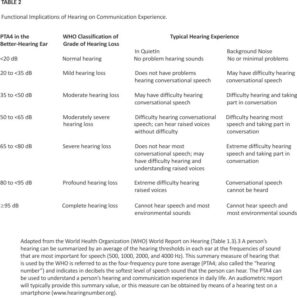
Evaluation of a patient’s hearing issues requires understanding that a person’s perception of hearing depends on four components: the quality of the incoming sound (e.g. because the speech signal becomes degraded in rooms with background noise or reverberant acoustics), the mechanical conduction of sound through the middle ear to the cochlea (i.e., conductive hearing), transduction of the acoustic signal into a neuroelectrical signal by the cochlea and transmission to the brain (i.e., sensorineural hearing), and decoding of the neural signal into meaning by the cortex (i.e., central auditory processing) (Table 1). When a patient notes problems with hearing, the cause can lie with any of these components, and in many cases, more than one component is affected before hearing problems become apparent.

The goal of the initial clinical evaluation is to evaluate the patient for readily treatable forms of conductive hearing loss or other forms of hearing loss that may warrant further evaluation with an otolaryngologist. Conductive forms of hearing loss that are readily treatable by the primary care clinician include otitis media and cerumen impaction and can be apparent based on history (e.g., acute onset with otalgia and aural fullness with an upper respiratory tract infection) or otoscopy (e.g., evidence of complete cerumen impaction in the ear canal). Symptoms and signs accompanying hearing loss that require further evaluation or consultation with an otolaryngologist include ear drainage, abnormal otoscopic examination, unremitting tinnitus, vertigo, fluctuating or asymmetric hearing, or sudden onset of hearing loss without evidence of a conductive cause (e.g., middle-ear effusion).
Sudden sensorineural hearing loss is one of the few forms of hearing loss that requires urgent evaluation with an otolaryngologist (ideally within 3 days after onset) because earlier diagnosis and intervention with glucocorticoids may improve the chances of hearing recovery. Sudden sensorineural hearing loss is a relatively uncommon event, with an annual incidence of 1 in 10,000 persons, and most commonly occurs in adults 40 years of age or older.12 As compared with a unilateral hearing loss from a conductive cause, patients with sudden sensorineural hearing loss will often report an acute, painless hearing loss in one ear that results in a near-complete inability to hear or understand speech in the affected ear.12
Multiple bedside screening methods for hearing loss exist, including whispered-voice and finger-rub tests. However, these measures produce widely varying levels of sensitivity and specificity13 and may be of limited usefulness depending on the suspected probability of a patient having age-related hearing loss. It is especially important to note that, given the progressive decline in hearing across the lifespan (Figure 1), some degree of age-related hearing loss can be inferred to be present regardless of screening results based on a patient’s age, symptoms indicative of hearing loss, and an absence of other clinical findings suggestive of other causes.
Confirmatory evaluation of hearing loss is performed with a referral to an audiologist. During an audiological evaluation, a patient’s hearing is tested with a calibrated audiometer in a sound-attenuating enclosure. The softest intensity of sound in decibels that a patient can reliably detect (i.e., the hearing threshold) is assessed across a range from 125 to 8000 Hz, with lower thresholds being indicative of better hearing. In children and young adults, thresholds across all frequencies will be close to 0 dB, but with progressive age-related declines in hearing, these thresholds will gradually increase, particularly for sounds at higher frequencies. The World Health Organization classifies hearing according to the average of a person’s hearing thresholds at the frequencies of sound that are considered to be the most important for speech (500, 1000, 2000, and 4000 Hz), termed the four-frequency pure tone average (PTA4) (Table 2). The PTA4 can be used by the clinician or patient to understand the functional implications of the patient’s level of hearing and appropriate management strategies (Figure 2). Other tests that are performed during the audiology examination (e.g., bone-conduction audiometry and speech understanding- ing) can also help to differentiate whether there may be a conductive or central auditory processing cause of hearing loss and to guide appropriate hearing rehabilitative options.
Evaluations of hearing that patients perform on their own are also increasingly available through digital applications. A recently adopted consumer technology industry standard14 for hearing-related technologies (e.g., smartphones and wireless earbuds) specifies how these applications can directly measure and report to users their PTA4 (also termed the “hearing number”; www.hearing-number.org), which can be tracked regularly. Such an approach aligns with broader trends toward empowering persons with direct access to metrics to monitor their health and increases awareness that hearing exists along a continuum that can be monitored and acted on for life, as is done for other health metrics (e.g., blood pressure).

MANAGEMENT
The primary clinical rationale for addressing age-related hearing loss is to enhance a person’s access to speech and other sounds in the auditory environment (e.g., music and audible alerts) to promote effective communication, engagement with daily activities, and safety. At present, there are no restorative therapies for age-related hearing loss and management of the condition is focused on hearing protection, adoption of communication strategies to optimize the quality of the incoming auditory signal (over competing background noise), and the use of hearing technologies such as hearing aids and cochlear implants (Figure 2). The prevalence of hearing aid use or cochlear implantation among persons who could benefit (as determined based on their audiologic hearing) remains very low. Among persons with hearing impairment in the United States, the prevalence of hearing aid use is below 20%4 and that of cochlear implantation is less than 5%.15 Reasons for the low rate of adoption are multifactorial and include such factors as stigma, poor accessibility to and affordability of hearing interventions, and the inability of hearing technologies to compensate fully for the degraded peripheral encoding of sound caused by age-related hearing loss.8
Hearing-protection strategies are focused on reducing noise exposure through movement away from or reduction in the volume of the sound source and by the use of hearing-protection devices (e.g., ear plugs) when needed. Communication strategies include encouraging persons to be face to face and at arm’s length when conversing and to reduce background noise. Face-to-face communication allows for both a clearer auditory signal to be received and for the listener to have visual access to facial expressions and lip movements that can aid in the central decoding of the speech signal.
Hearing aids remain the primary treatment option for age-related hearing loss. Hearing aids amplify sound, and more advanced hearing aids can also increase the signal-to-noise ratio of the desired target sound (e.g., amplifying a speaker’s voice over the background noise) through directional microphones and digital signal processing, which is critical for improving communication in noisy settings. Before 2022, hearing aids in the United States could only be purchased with a hearing professional (typically an audiologist or hearing instrument specialist) as an intermediary.
Beginning on October 17, 2022, the Food and Drug Administration enacted new regulations allowing for the sale of over-the-counter hearing aids that would be available to consumers, without a hearing professional as an intermediary.16 These over-the-counter hearing aids are intended for adults with perceived mild-to-moderate levels of hearing loss with PTA4 values generally less than 60 dB, which encompasses 90 to 95% of all persons with hearing loss (Figure 1). In contrast, prescription hearing aids have higher levels of sound output and can be used by adults with more severe levels of hearing loss but are only available with a hearing professional as an intermediary. The cost of these over-the-counter hearing aids once the market is mature is expected to be on par with higher-quality wireless earbuds that often range from $100 to $300 in the United States. Over-the-counter hearing aids may eventually become indistinguishable from wireless earbuds as hearing-aid features become routinely incorporated into these devices.
A previous Cochrane systematic review concluded that hearing aids in adults improve outcomes of both hearing-specific and general health-related quality of life.17 One recently published randomized trial (Aging and Cognitive Health Evaluation in Elders [ACHIEVE]) investigated the distal effects of hearing intervention (e.g., hearing aids and related audiologic services to support technology use) as compared with health education (control) on reducing 3-year cognitive decline in adults 70 to 84 years of age with hearing loss.18 In the primary analysis of the total ACHIEVE cohort, hearing intervention did not reduce 3-year cognitive decline as compared with control. However, a prespecified sensitivity analysis showed that in the trial population of participants who were at increased baseline risk for cognitive decline, hearing intervention reduced cognitive change by 48% for 3 years (change in 3-year global cognitive decline, −0.211 SD units in the intervention group vs. −0.402 SD units in the control group). In contrast, no effect of hearing intervention was observed in the trial population consisting of healthy volunteers at decreased baseline risk for cognitive decline. Continued follow-up of the ACHIEVE cohort beyond 3 years and other longer-term studies will be needed to further understand the potential effects of hearing intervention on reducing cognitive decline and the risk of dementia.
Persons who have hearing loss of greater severity (PTA4 values generally ≥60 dB) and who continue to have difficulty with understanding speech despite the use of hearing aids may be candidates for a cochlear implant. A cochlear implant is a neuroprosthetic device that encodes sounds and directly stimulates the cochlear nerve. It is implanted by an otolaryngologist during outpatient surgery that takes approximately 2 hours. A period of 6 to 12 months is needed after implantation for the patient to become accustomed to hearing with the implant and perceiving the neuroelectrical stimuli as meaningful language and sound. Although there is variance in hearing results after a cochlear implant, the improvement in speech understanding and communication is often described as “life-changing” by many adults who had long struggled to adequately communicate.19 Potential candidates for a cochlear implant should be referred to a cochlear implant centre or an otolaryngologist who specializes in cochlear implantation.
Areas of Uncertainty
Age-related hearing loss results from the combined effects of multiple etiologic factors occurring over one’s lifetime.1,2 Whether pharmacologic or genetic therapies could be feasibly used to reduce the progression of age-related hearing loss or to restore hearing function is an active area of academic and industry research, but efforts have been largely unsuccessful to date.20 Although several mechanisms have been proposed through which age-related hearing loss could adversely affect health, one provocative mechanism suggests that impaired hearing and diminished auditory afferents may directly affect brain function and structure.21 Understanding whether existing hearing rehabilitative technologies could modify these effects and help support brain health will be important for optimizing future intervention strategies.
Guidelines
In 2021, the U.S. Preventive Services Task Force determined that there is insufficient evidence to assess the benefits and harms of screening for hearing impairment in asymptomatic adults 50 years or older.22 Clinicians were advised to use their clinical judgment about conducting hearing tests in patients who have symptoms of hearing loss or who have raised concerns about their hearing. To my knowledge, no other clinical practice guidelines on age-related hearing loss are currently available.
CONCLUSIONS
The patient described in this vignette presents with a history consistent with age-related hearing loss. After evaluating for and ruling out a potential conductive cause or need for otolaryngology referral, I would presume that some degree of age-related hearing loss is present. I would counsel the patient and his wife about how age-related hearing loss as well as other factors can affect perceptions of hearing and how hearing strategies and technologies can improve communicative and social functioning and potentially have distal effects on supporting cognitive health. Depending on the patient’s preference, I would refer the patient to an audiologist for a formal diagnostic evaluation and counselling about treatment options or to a well-regarded retail centre that sells hearing aids (e.g., Costco). If the patient is familiar with using technology, I would provide resources to help the patient learn how to do a self-test of hearing with a smartphone. I would also discuss the availability of over-the-counter hearing aids and explain that retail choices for over-the-counter hearing aids and support options for these technologies are expected to rapidly increase in the next 2 to 3 years as the market for over-the-counter hearing aids matures in the United States.
REFERENCES
1. Pickles JO. An introduction to the physiology of hearing. Bingley, United Kingdom: Emerald Group Publishing, 2008.
2. Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med 2017;377:2465-2473.
3. World report on hearing. Geneva: World Health Organization, 2021 (https://www.who.int/ publications/i/item/9789240020481).
4. Haile LM, Orji AU, Reavis KM, et al. Hearing loss prevalence, years lived with disability, and hearing aid use in the United States From 1990 to 2019: findings from the global burden of disease study. Ear Hear 2024; 45:257-267.
5. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018;144:115-126.
6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413-446.
7. Reed NS, Altan A, Deal JA, et al. Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Head Neck Surg 2019;145:27-34.
8. Blazer DG, Domnitz SB, Liverman CT, National Academies of Sciences Engineering and Medicine. Hearing health care for adults: priorities for improving access and affordability. Washington, DC: National Academies Press, 2016 (https://www.ncbi.nlm. nih.gov/books/NBK367633/).
9. Lin FR, Albert M. Hearing loss and dementia — who is listening? Aging Ment Health 2014;18: 671-673.
10. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev 2015;23: 154-166.
11. Peelle JE, Wingfield A. The neural consequences of age-related hearing loss. Trends Neurosci 2016;39:486-497.
12. Rauch SD. Idiopathic sudden sensorineural hearing loss. N Engl J Med 2008;359:833-840.
13. Boatman DF, Miglioretti DL, Eberwein C, Alidoost M, Reich SG. How accurate are bedside hearing tests? Neurology 2007; 68:1311-1314.
14. American National Standards Institute, Consumer Technology Association. Four frequency pure tone average testing methodology and reporting metrics for consumer-facing hearing solutions. October 2023 (https://shop.cta.tech/products /four-frequency-pure-ton-average-testing-methodo- logy-and-reporting-metrics-for-consumer-facing-hearing-solutions-cta-2118).
15. Nassiri AM, Sorkin DL, Carlson ML. Current estimates of cochlear implant utilization in the United States. Otol Neurotol 2022;43(5):e558-e562.
16. Lin FR, Chadha S. Over-the-counter hearing aids — using regulatory policy to improve public health. N Engl J Med 2023;388:2117-2119.
17. Ferguson MA, Kitterick PT, Chong LY, Edmondson-Jones M, Barker F, Hoare DJ. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev 2017;9: CD012023-CD012023.
18. Lin FR, Pike JR, Albert MS, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multi-centre, randomised controlled trial. Lancet 2023;402:786-797.
19. Buchman CA, Gifford RH, Haynes DS, et al. Unilateral cochlear implants for severe, profound, or moderate sloping to profound bilateral sensorineural hearing loss: a systematic review and consensus statements. JAMA Otolaryngol Head Neck Surg 2020;146:942-953.
20. Schilder AGM, Su MP, Blackshaw H, et al. Hearing protection, restoration, and regeneration: an overview of emerging therapeutics for inner ear and central hearing disorders. Otol Neurotol 2019;40:559-570.
21. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and cognitive decline: MRI and cellular evidence. Ann N Y Acad Sci 2021;1500:17-33.
22. Krist AH, Davidson KW, Mangione CM, et al. Screening for hearing loss in older adults: US Preventive Services Task Force Recommendation Statement. JAMA 2021; 325: 1196-1201.
Credits: Lin FR· Age-Related Hearing Loss. N Engl J Med. 2024 Apr 25; 390(16):1505-1512.











