Dhoot, Dilsher S. MD*; Moini, Hadi PhD†; Reed, Kimberly OD†; Silva, Fabiana Q. MD†; Berliner, Alyson MD, PhD†; Du, Weiming MS†; Sharma, Sumit MD
Abstract
Purpose:
To characterize diabetic macular edema (DME) incidence in fellow eyes of patients treated for DME in the study eye.
Methods:
This post hoc analysis of VISTA/VIVID data evaluated fellow eyes without DME at baseline through Week 100. Diabetic macular edema presence in the fellow eye was inferred by investigator-reported DME adverse events and the use of DME treatments.
Results:
Over 100 weeks, 44.9%, 44.2%, and 42.9% of fellow eyes developed DME in the intravitreal aflibercept injection 2 mg every 4 weeks (n = 245), intravitreal aflibercept injection 2 mg every 8 weeks (n = 258), and laser control (n = 252) groups, respectively. The mean time for DME development in combined treatment groups was ∼6 months. Multivariable regression analysis confirmed patients with shorter diabetes duration (hazard ratio per 10-year decrease, 1.16; 95% confidence interval, 1.03–1.30; P = 0.0160) and thicker baseline study eye central subfield thickness (hazard ratio per 10-µm increase, 1.01; 95% confidence interval, 1.01–1.02; P = 0.0002) were at higher risk of developing DME in the fellow eye.
Conclusion:
Among patients with DME in one eye at baseline, almost half developed DME in the fellow eye over 2 years. The shorter duration of diabetes and thicker study eye central subfield thickness were predictors of DME development in the fellow eye.
Center-involving diabetic macular edema (DME) is the most common cause of vision loss in patients with diabetic retinopathy (DR) and is characterized by microvascular damage, resulting in exudation and accumulation of extracellular fluid in the macula with loss of central vision.1,2 In 2010, there were 20.6 million adults with DR worldwide with more than 700,000 individuals aged 40 or older in the United States estimated to be affected by DME, numbers that are likely to increase with the increasing prevalence of diabetes.3,4
In addition to causing reduced visual acuity, DME can also negatively affect the vision-related quality of life.5 In a recent retrospective analysis of the Intelligent Research in Sight registry, ∼51.2% of patients with treatment-naive or previously treated DME had bilateral disease.6 Additionally, Man et al7 reported a 17% reduction in vision-related quality of life when a single eye was affected, which worsened to 22% in bilateral DME.
Early diagnosis remains the key strategy for reducing the risk of DME-related blindness and preserving the vision-related quality of life.8 However, despite the high burden of bilateral disease, there is little evidence to guide management in the fellow eye for individuals who initially have DME in only one eye. Identifying the expected timeline for and factors predictive of DME development in the fellow eye may help clinicians identify at-risk patients who need more frequent monitoring, and help set patient expectations about their risks of disease progression.
Intravitreal aflibercept injection (IAI) significantly improved visual and anatomic outcomes compared with laser photocoagulation in the Phase 3 VISTA and VIVID trials in patients with DME through Week 100.9 Herein, a post hoc analysis of the data from VISTA and VIVID was performed to characterize the occurrence and identify factors predictive of DME development in the fellow eyes of patients with unilateral DME treated with IAI or laser therapy, through 100 weeks.
Methods
Study Design
The study designs of the VISTA (ClinicalTrials.gov: NCT01363440) and VIVID (ClinicalTrials.gov: NCT0133 1681) clinical trials were previously reported.10 Briefly, VISTA and VIVID were similarly designed, randomized, double-masked, active-controlled Phase 3 clinical trials in adult patients with Type 1 or 2 diabetes who had clinically significant DME with central involvement. The studies were conducted at 127 sites across Australia, Europe, Japan, and the United States in accordance with the International Council for Harmonisation guidelines, the Health Insurance Portability and Accountability Act of 1996, and the Declaration of Helsinki. All patients provided written informed consent and institutional review board/ethics committee approval was obtained.
Adult patients with Type 1 or 2 diabetes mellitus who demonstrated DME with central involvement (defined as retinal thickening involving the 1-mm central subfield) in the study eye were included if best-corrected visual acuity (BCVA) was between 73 and 24 letters (Snellen equivalent, 20/40–20/ 320). In both trials, one eye per patient was enrolled, with the untreated eye referred to as the fellow eye. Study eyes were randomized 1:1:1 to receive IAI 2 mg every 4 weeks (2q4), IAI 2 mg every 8 weeks (2q8) after five initial monthly doses (from baseline to Week 16) with sham injections on nontreatment visits or macular laser photocoagulation at baseline and sham injections at every visit (laser control group). Fellow eyes were treated at the investigator’s discretion.
Post Hoc Analysis
Integrated data from the VISTA and VIVID clinical trials were used in this analysis. Patients in the safety analysis set (defined as patients who received ≥1 study treatment: IAI or laser control) with no DME in the fellow eye during the baseline period were included. The baseline period was defined as 6 weeks before baseline through 4 weeks after the first study eye treatment. Fellow eye optical coherence tomography imaging was not available for this analysis. Diabetic macular edema presence in the fellow eye after the baseline period was determined based on the incidence of adverse events related to DME and the reported use of treatments for DME (anti–vascular endothelial growth factor [VEGF] agents or laser). Adverse events considered related to DME included the presence of diabetic retinal edema, DR without proliferative DR, or vitreous hemorrhage but treated with anti–VEGFs or steroids (given that at the time of the study [May 2011–May 2014] anti–VEGFs were almost exclusively used for the treatment of DME), or cystoid macular edema in the absence of cataract history within 30 days. The primary objective of this analysis was to evaluate the cumulative incidence of and time to DME development in the fellow eye. The relationship between baseline characteristics and DME occurrence in the fellow eye was examined. Cumulative rates of DME development by specific baseline risk factors were also assessed.
Statistical Analysis
The cumulative incidence of DME occurrence was evaluated using the Kaplan–Meier method. Log-rank test was used to assess the difference between the two Kaplan–Meier curves. The relative risks between subgroups were estimated using Cox proportional hazards analysis. The time at risk for each patient was defined as the minimum of the time from randomization to the first of any of the following: 1) the date a patient discontinued the study; 2) the date of the first episode of the evaluated event; or 3) the end of the study. The time at risk was expressed as person-years at risk (PYR), with the rate expressed as the number of events/PYR. The relative hazard was determined as the ratio of the rate in one subgroup to the rate in the other subgroups.
The mean differences between the treatment groups in time to DME development in the fellow eye were evaluated using t-tests. A univariable analysis using a Cox regression model was conducted to examine the relationship between baseline characteristics and DME incidence in the fellow eye. Baseline variables included in the analysis were age, sex, ethnicity, body mass index, history of smoking, and diabetes duration; BCVA, Diabetic Retinopathy Severity Scale (DRSS) score, and central subfield thickness (CST) in the study eye; comorbid hypertension, anxiety, depression, dyslipidemia, and hyperlipidemia; and concomitant use of antidepressants and insulin. Factors found to be significant in the univariable analysis were tested further in the multivariable analysis using stepwise selection. The hazard ratio (HR) was estimated using a Cox regression model. Cumulative incidence of DME development in the fellow eye and its association with specific baseline factors, confirmed through multivariable analysis, were examined and reported by tertiles (using pooled data from across the treatment groups). A P value of <0.05 was considered nominally significant for this analysis. Analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Baseline Demographic and Disease Characteristics
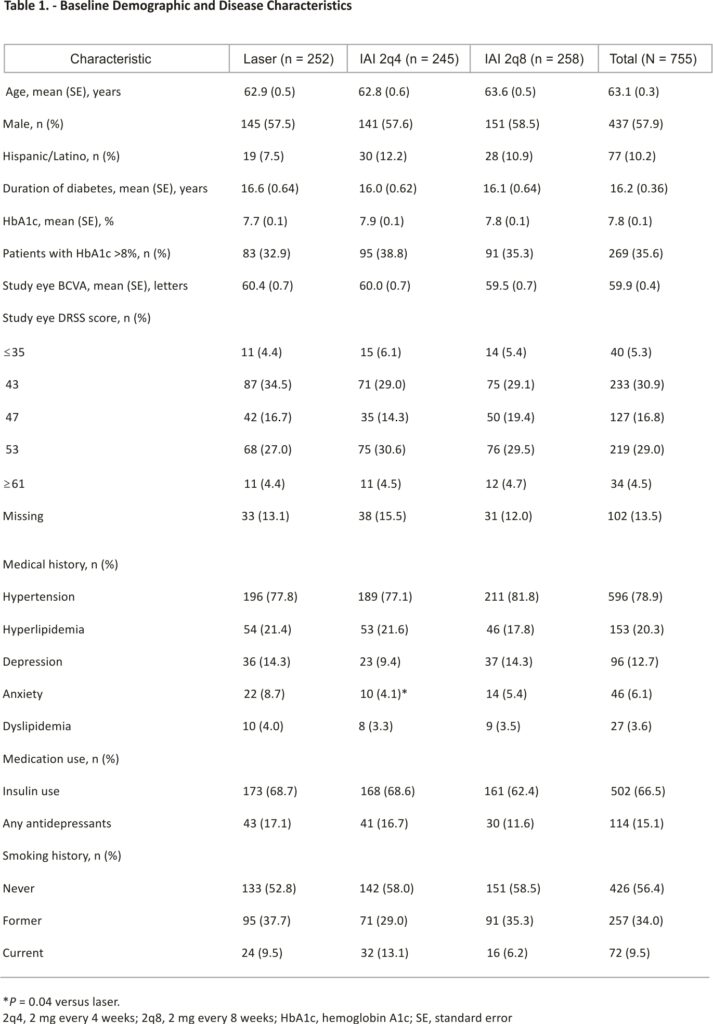
Of the 865 patients in the VISTA and VIVID safety analysis set, 755 had no DME in the fellow eye at baseline and were included in this analysis (IAI 2q4 group, n = 245; IAI 2q8 group, n = 258; laser group, n = 252) (see Figure 1, Supplemental Digital Content, https:// links.lww.com/IAE/B842). At baseline, the patient demographic and disease characteristics across the three treatment groups were similar (Table 1). Overall, 57.9% of patients were men, the mean hemoglobin A1c was 7.8%, and the mean duration of diabetes was 16.2 years. Comorbid hypertension and insulin use were reported in 78.9% and 66.5% of patients, respectively. The mean BCVA in the study eye was 59.9 letters, and 45.8% of patients had a moderately severe-to-severe DRSS score (47–53).
Diabetic Macular Edema Incidence in the Fellow Eye Through Week 100
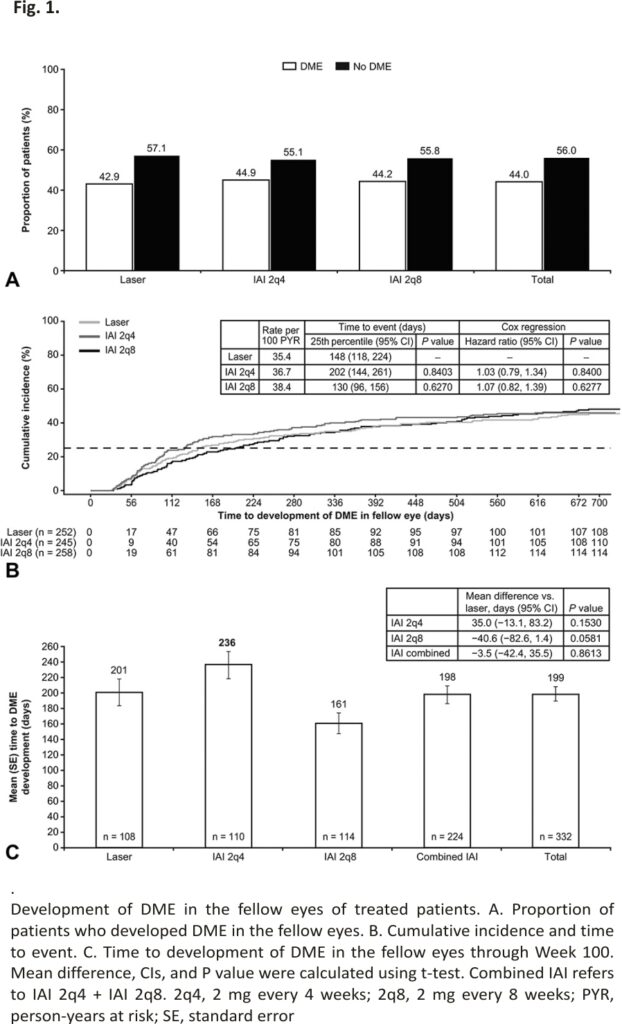
Of the 755 fellow eyes without DME at baseline, 332 developed DME through Week 100. The presence of DME was inferred in 204 of 332 fellow eyes (61.5%) based on adverse events, including 95 with diabetic retinal edema, 74 with DR without proliferative DR or vitreous hemorrhage but treated with anti–VEGF or steroids, and 35 with cystoid macular edema in the absence of cataract history within 30 days. The presence of DME in the remaining 128 of 332 fellow eyes (38.5%) was inferred based on reported treatment with laser only.
The proportions of patients who developed DME in the fellow eye through Week 100 were 44.9%, 44.2%, and 42.9% in the IAI 2q4, IAI 2q8, and laser groups, respectively (Figure 1A). The risk of DME development in the fellow eye was similar in the IAI groups compared with laser control: HRs were 1.03 (95% confidence interval [CI], 0.79–1.34; P = 0.84) for IAI 2q4 versus laser and 1.07 (95% CI, 0.82–1.39; P = 0.62) for IAI 2q8 versus laser (Figure 1B). Among patients who developed DME in the fellow eye, the mean time to DME development for the IAI and laser groups ranged between 161 and 236 days (P values for between-group comparisons were all >0.05) (Figure 1C). For all groups combined, the mean (median) time to DME development in fellow eyes was 199 (134) days (∼6 months).
Baseline Characteristics Predicting Diabetic Macular Edema Development in the Fellow Eye
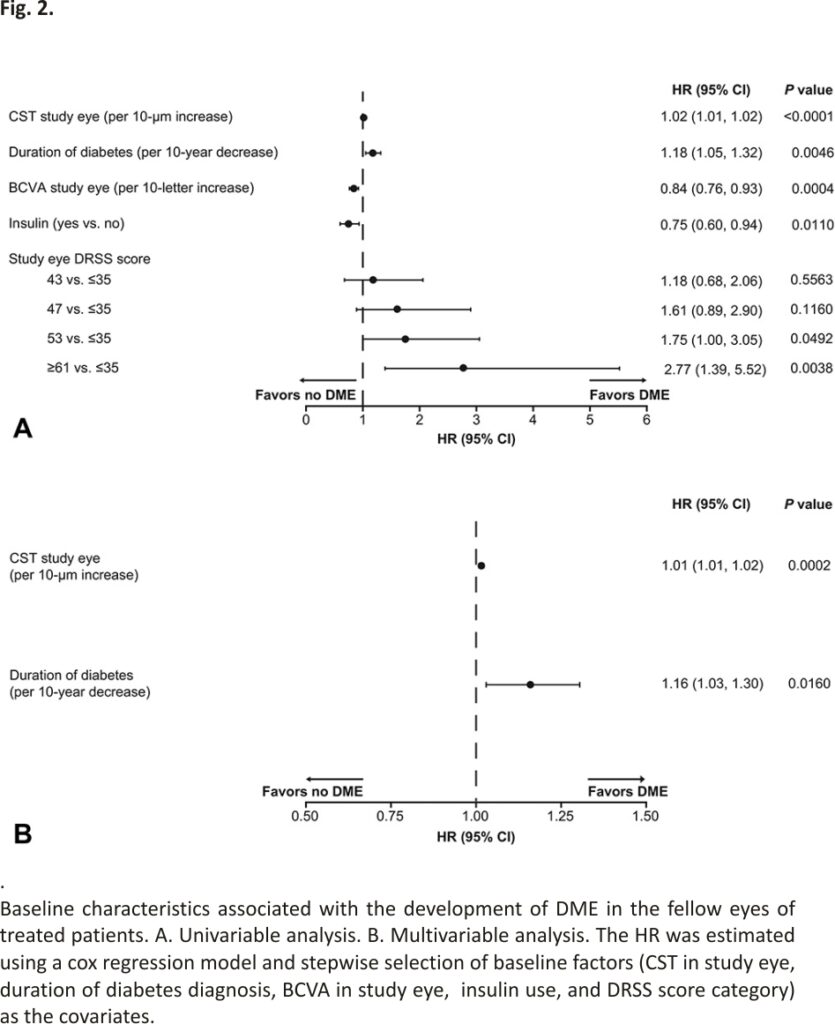
In the univariable analysis, the likelihood of developing DME in the fellow eye increased with thicker CST in the study eye (HR per 10-µm increase, 1.02; 95% CI, 1.01–1.02; P < 0.0001), shorter duration of diabetes (HR per 10-year decrease, 1.18; 95% CI, 1.05– 1.32; P = 0.0046), and higher study eye DRSS score (53 vs. ≤35: HR, 1.75; 95% CI, 1.00–3.05; P = 0.0492; and ≥61 vs. ≤35: HR, 2.77; 95% CI, 1.39–5.52; P = 0.0038). Better BCVA in the study eye (HR per 10-letter increase, 0.84; 95% CI, 0.76–0.93; P = 0.0004) and insulin use (HR, 0.75; 95% CI, 0.60–0.94; P = 0.0110) were associated with a lower risk of developing DME in the fellow eye (Figure 2A). The multivariable analysis confirmed that thicker CST in the study eye (HR, 1.01; 95% CI, 1.01–1.02; P = 0.0002) and shorter duration of diabetes (HR, 1.16; 95% CI, 1.03–1.30; P = 0.0160) were associated with increased risk of developing DME in the fellow eye (Figure 2B).
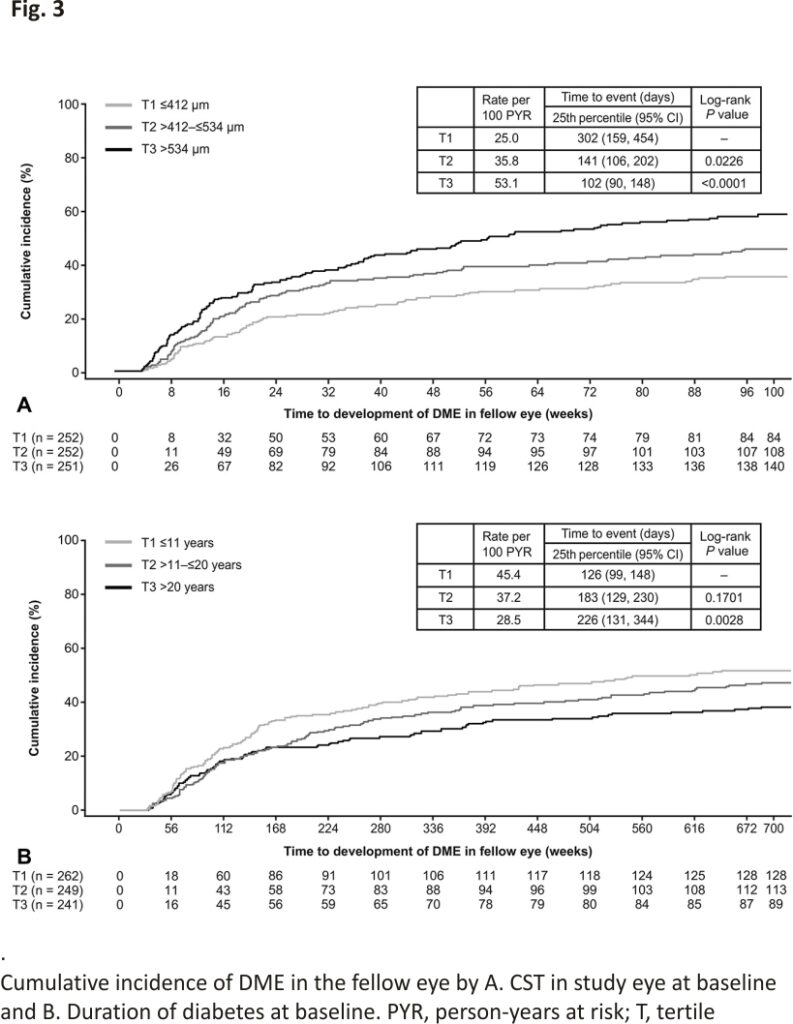
Kaplan–Meier analysis of baseline CST in the study eye by tertiles (Tertile [T] 1, ≤412 µm; T2, >412 to ≤534 µm; T3, >534 µm) indicated that the cumulative incidence of DME in the fellow eye over 100 weeks was significantly higher in the tertiles with thicker baseline CST (T2 vs. T1, 45.4% vs. 35.2%; log-rank P = 0.0226; and T3 vs. T1, 58.4% vs. 35.2%; log-rank P < 0.0001) (Figure 3A). Patients with baseline CST >412 µm to ≤534 µm (25th percentile, 141 days) or >534 µm (25th percentile, 102 days) developed DME in the fellow eye sooner than patients with baseline CST ≤412 µm (25th percentile, 302 days) (Figure 3A).
A Kaplan–Meier analysis based on diabetes duration (T1, ≤11 years; T2, >11 to ≤20 years; T3, >20 years) showed that the cumulative incidence of DME in the fellow eye decreased with increasing duration of diabetes (T3 vs. T1, 38.6% vs. 52.1%; log-rank P = 0.0028; and T2 vs. T1, 47.6% vs. 52.1%; log-rank P = 0.1701) (Figure 3B). Moreover, patients with longer diabetes duration tended to develop DME in the fellow eye later in the course of the disease than patients with shorter diabetes duration (25th percentile T3, 226 days; 25th percentile T2, 183 days; 25th percentile T1, 126 days) (Figure 3B).
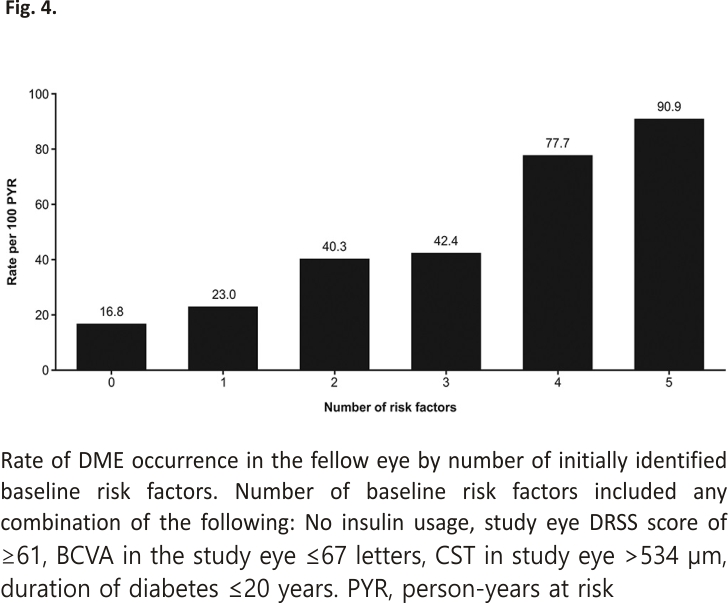
The risk of developing DME in the fellow eye increased with the increasing number of baseline risk factors that were identified in the univariable analysis: the DME incidence rate in the fellow eyes of patients with all five identified baseline risk factors was 90.9 per 100 PYR, compared with 23.0 and 40.3 per 100 PYR for patients with one and two risk factors, respectively (Figure 4).
Discussion
The VISTA and VIVID studies evaluated the safety and efficacy of IAI versus laser in the treatment of DME. In the present VISTA and VIVID post hoc analysis, among patients with DME in a single (study) eye, 44% developed DME in the fellow eye by Week 100, with similar incidences across IAI- and laser-treated groups and a mean time to onset of ∼6 months for all groups combined.
Diabetic macular edema incidence in the fellow eye has not been well studied. Varma et al11 reported an 11.5% incidence of DME in the fellow eyes of Latino patients with diabetes in a 4-year analysis; however, their estimate was based on a population that included patients without DR. To the best of our knowledge, this is the first study to evaluate the rate and time to progression of bilateral DME in patients with documented DR and unilateral DME.
Several baseline risk factors for DME development in the fellow eye were identified in the univariable analysis, including thicker CST and higher DRSS score in the study eye, and shorter duration of diabetes. Of these, thicker CST in the study eye and shorter duration of diabetes was confirmed in the multivariable analysis and were the key drivers in predicting DME development in the fellow eye. To our knowledge, no previous studies have investigated the correlation between CST in the study eye and DME development in the fellow eye. Our analyses indicate thicker CST in the study eye was associated with increased DME incidence in the fellow eye. A potential explanation for our findings is that patients with thicker CST may have more severe disease and are therefore at higher risk of developing DME in the fellow eye. The correlation between macular thickening and DR severity, and the increased prevalence of DME in patients with severe disease, has been well described and may support the potential rationale for our findings.12–14
The relationship between the duration of diabetes and the development of DME has been evaluated previously, with controversial results. Klein et al15 found no linear association between these parameters in patients with Type 1 diabetes, whereas Varma et al11 found a significant increasing trend in DME incidence in the first eye with increasing duration of diabetes (P = 0.004); however, this trend was not observed for DME incidence in the fellow eye (P = 0.76). The present study demonstrated an increased DME incidence in the fellow eye of patients with a shorter duration of diabetes in univariable and multivariable analyses. The reasons for this association are unclear. A potential explanation is that patients who develop unilateral DME in the early years after being diagnosed with diabetes may be more susceptible to progressing to bilateral disease, whereas patients who develop unilateral DME later in the course of the disease may be less likely to progress to DME in the fellow eye. The lack of differentiation in the present study between patients with Type 1 diabetes (typically diagnosed at a younger age) and Type 2 diabetes (typically diagnosed at an older age) may also support this speculative association. This possibility could not be evaluated in the present study, however, because of the small number of patients with Type 1 diabetes.
The risk of developing DME in the fellow eye increased with each additional risk factor identified in the univariable analysis. A shorter duration of diabetes, thicker CST, higher DRSS score, lower BCVA in the study eye, and not receiving insulin also contributed to increased risk. These findings suggest that patients with >1 of these risk factors may benefit from more aggressive monitoring for DME development in the fellow eye.
Study strengths include the use of data from randomized-controlled clinical trials with fixed-dosing treatment schedules, large sample sizes, and long-term follow-up through Week 100. Limitations include those inherent to a post hoc analysis. Since the VISTA and VIVID clinical trials limited enrollment to a single eye, detailed data related to DME onset and changes in vision for the fellow eye were not available for analysis, including OCT and anatomy data. For this analysis, DME in the fellow eye was determined indirectly based on the incidence of reported adverse events, concomitant medication use, or procedures. The absence of anatomic OCT data in the fellow eye may have resulted in under-reporting of DME; however, one would expect this to be uniform across all three groups. Since the treatment decision in the fellow eye was at the investigator’s discretion, this may represent the real-world likelihood of needing to treat the fellow eye. These patients were followed very closely because they were in a clinical trial and investigators were able to obtain imaging data from the fellow eye as needed. Data could not be analyzed by diabetes type because the majority of (92%) patients in the VISTA study had Type 2 diabetes and diabetes type was not specified in the VIVID study. An additional limitation is that the studies only included patients who were eligible and agreed to participate in the clinical trials, excluding patients on hemodialysis, those with uncontrolled diabetes mellitus, and those with active proliferative DR. Thus, these data are only generalizable to this study population. Nevertheless, data from this analysis suggest further research is merited to explore the association of additional risk factors with DME development in the fellow eyes of patients with DME in one eye.
In VISTA and VIVID, nearly half of the patients with DME in only one eye at baseline developed DME in the fellow eye by Week 100. From a health-related quality-of-life perspective, this finding needs to be considered as individuals with unilateral disease tend to rely on their fellow eye for daily activities. When the fellow eye is also compromised, the ability of patients to perform regular activities becomes severely impaired, resulting in an important impact on their quality of life.7 Diabetic macular edema development in the fellow eye was more likely in patients who had thicker CST in the study eye and shorter diabetes duration. Identification of such risk factors for DME development in the fellow eye and further investigation of additional factors are crucial to enable early diagnosis and better management of at-risk patients.
Acknowledgments
The authors thank the study investigators and patients involved in the study. The medical writing support was provided by Rhutika Dessai, MSc, of Core (London, United Kingdom), in accordance with Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288), and funded by Regeneron Pharmaceuticals, Inc. The authors participated in the study design and collection, analysis, and interpretation of data. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author Information
*California Retina Consultants/Retina Consultants of America, Santa Barbara, California;
†Regeneron Pharmaceuticals, Inc., Tarrytown, New York; and
‡Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio.
Supported by Regeneron Pharmaceuticals, Inc. (Tarrytown, New York). The sponsor participated in the design and conduct of the study, analysis of the data, and preparation of this manuscript.
D. S. Dhoot is a consultant for Regeneron Pharmaceuticals, Inc., Genentech, Allergan, Alimera, Santen, Allegro, Bayer, Eyepoint Pharmaceuticals, Novartis, and Notal Vision. H. Moini, K. Reed, F. Q. Silva, A. Berliner, and W. Du are employees and stockholders in Regeneron Pharmaceuticals, Inc. S. Sharma is a consultant for Alimera, AbbVie, Bausch and Lomb, Clearside, Eyepoint, IONIS, Genentech/Roche, Regeneron Pharmaceuticals, Inc., and REGENXBIO; and reports contracted research support from Gilead, Genentech/Roche, IONIS, and Santen.
All authors contributed to the concept, design, data collection, interpretation, analysis, and drafting of the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.retinajournal.com).
References
1. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 1999;14:223–232.
2. Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab 2013;4:151–169.
3. International Diabetes Federation. Clinical Practice Recommendations for Managing Diabetic Macular Edema; 2019. Available at: https://www.idf.org/e-library/guide lines/161-dme-clinical-practice-recommendations.html.
4. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 2014;132:1334–1340.
5. Giocanti-Auregan A, Tadayoni R, Grenet T, et al. Estimation of the need for bilateral intravitreal anti-VEGF injections in clinical practice. BMC Ophthalmol 2016;16:142.
6. Cantrell RA, Lum F, Chia Y, et al. Treatment patterns for diabetic macular edema: an Intelligent Research in Sight (IRIS®) registry analysis. Ophthalmology 2020;127: 427–429.
7. Man REK, Fenwick EK, Sabanayagam C, et al. Differential impact of unilateral and bilateral classifications of diabetic retinopathy and diabetic macular edema on vision-related quality of life. Invest Ophthalmol Vis Sci 2016;57:4655–4660.
8. National Eye Institute, National Institutes of Health, and US Department of Health and Human Services. Diabetic Retinopathy: What You Should Know; 2020. Available at: https://www.nei. nih.gov/learn-about-eye-health/ resources-for-health-educators /outreach-materials/diabetic-retinopathy-what-you-should-know.
9. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122:2044–2052.
10. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121:2247–2254.
11. Varma R, Choudhury F, Klein R, et al. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol 2010;149:752–761.
12. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801 –1815.
13. Stewart M, Browning D, Lee C. Diabetic macular edema: evidence-based management. Indian J Ophthalmol 2018;66:1736–1750.
14. Lattanzio R, Brancato R, Pierro L, et al. Macular thickness measured by optical coherence tomography (OCT) in diabetic patients. Eur J Ophthalmol 2002;12:482–487.
15. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 2009;116:497–503.
Keywords:
best-corrected visual acuity; diabetic macular edema; central subfield thickness; fellow eye; diabetes duration; vascular endothelial growth factor
CREDITS: Dhoot, Dilsher S. MD*; Moini, Hadi PhD†; Reed, Kimberly OD†; Silva, Fabiana Q. MD†; Berliner, Alyson MD, PhD†; Du, Weiming MS†; Sharma, Sumit MD‡. INCIDENCE OF NEW DIABETIC MACULAR EDEMA IN FELLOW EYES OF PATIENTS IN THE VISTA AND VIVID STUDIES. Retina 43(2):p 254-262, February 2023. | DOI: 10. 1097/IAE.0000000000003658