MeiLan K. Han, M.D., Wen Ye, Ph.D., Di Wang, M.S., Emily White, M.S., Mehrdad Arjomandi, M.D., Igor Z. Barjaktarevic, M.D., Stacey-Ann Brown, M.D., Russell G. Buhr, M.D., Alejandro P. Comellas, M.D., Christopher B. Cooper, M.D., Gerard J. Criner, M.D., Mark T. Dransfield, M.D., et al., for the RETHINC Study Group*
Abstract
BACKGROUND
Many persons with a history of smoking tobacco have clinically significant respiratory symptoms despite an absence of airflow obstruction as assessed by spirometry. They are often treated with medications for chronic obstructive pulmonary disease (COPD), but supporting evidence for this treatment is lacking.
METHODS
We randomly assigned persons who had a tobacco-smoking history of at least 10 pack-years, respiratory symptoms as defined by a COPD Assessment Test score of at least 10 (scores range from 0 to 40, with higher scores indicating worse symptoms), and preserved lung function on spirometry (ratio of forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ≥0.70 and FVC ≥70% of the predicted value after bronchodilator use) to receive either indacaterol (27.5 μg) plus glycopyrrolate (15.6 μg) or placebo twice daily for 12 weeks. The primary outcome was at least a 4-point decrease (i.e., improvement) in the St. George’s Respiratory Questionnaire (SGRQ) score (scores range from 0 to 100, with higher scores indicating worse health status) after 12 weeks without treatment failure (defined as an increase in lower respiratory symptoms treated with a long-acting inhaled bronchodilator, glucocorticoid, or antibiotic agent).
RESULTS
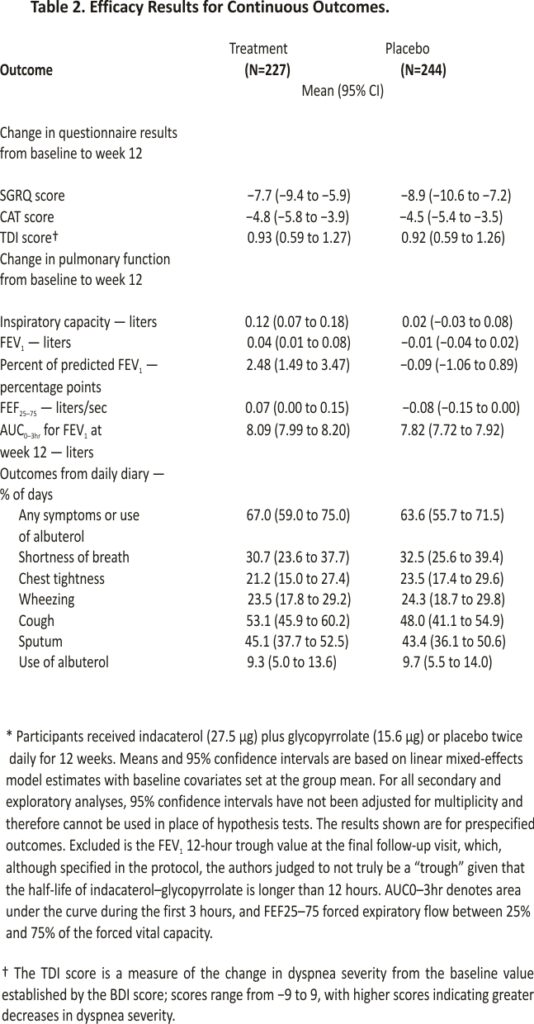
A total of 535 participants underwent randomization. In the modified intention-to-treat population (471 participants), 128 of 227 participants (56.4%) in the treatment group and 144 of 244 (59.0%) in the placebo group had at least a 4-point decrease in the SGRQ score (difference, −2.6 percentage points; 95% confidence interval [CI], −11.6 to 6.3; adjusted odds ratio, 0.91; 95% CI, 0.60 to 1.37; P=0.65). The mean change in the percent of predicted FEV1 was 2.48 percentage points (95% CI, 1.49 to 3.47) in the treatment group and −0.09 percentage points (95% CI, −1.06 to 0.89) in the placebo group, and the mean change in the inspiratory capacity was 0.12 liters (95% CI, 0.07 to 0.18) in the treatment group and 0.02 liters (95% CI, −0.03 to 0.08) in the placebo group. Four serious adverse events occurred in the treatment group, and 11 occurred in the placebo group; none were deemed potentially related to the treatment or placebo.
CONCLUSIONS
Inhaled dual bronchodilator therapy did not decrease respiratory symptoms in symptomatic, tobacco-exposed persons with preserved lung function as assessed by spirometry. (Funded by the National Heart, Lung, and Blood Institute and others; RETHINC Clinical- Trials.gov number, NCT02867761. opens in a new tab.)
Chronic obstructive pulmonary disease (COPD) is defined by a reduced ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) after bronchodilator use.1 However, we previously found that some tobacco-exposed persons who have preserved lung function as assessed by spirometry report having substantial respiratory symptoms, activity limitation, and exacerbations, similar to those in persons with airflow obstruction measured on spirometry.2 These tobacco-exposed persons with preserved lung function on spirometry who have respiratory symptoms as defined by a COPD Assessment Test (CAT) score of 10 or greater (scores range from 0 to 40, with higher scores indicating worse symptoms) also have airway-wall thickening and higher sputum mucin concentrations than nonsymptomatic persons.3 In the COPDGene cohort, a significant percentage of tobacco-exposed persons with preserved lung function on spirometry were noted to have respiratory impairments and abnormalities on computed tomography (CT), such as emphysema and air trapping.4 In the CanCOLD cohort, exacerbations among tobacco-exposed persons with preserved lung function on spirometry were associated with missed social activities, missed work for income, and an inability to do housework, which suggests that they constitute a real-life clinical burden.5
Many symptomatic tobacco-exposed persons with preserved lung function on spirometry are treated with COPD medications, including inhaled bronchodilators and glucocorticoids.2 Because spirometry is infrequently performed in primary care,6,7 it is unclear whether physicians believe they are treating COPD or whether they believe COPD medications are effective for these patients. Regardless, randomized trials to guide treatment in this patient population are lacking.
In response to this evidence gap, we hypothesized that persons who currently or formerly smoked cigarettes with at least a 10-pack-year history and who have clinically significant respiratory symptoms despite also having preserved lung function on spirometry (i.e., FEV1:FVC ≥0.70 and FVC ≥70% of the predicted value) would benefit from treatment with inhaled bronchodilators. We tested this hypothesis in a randomized trial using an inhaled dual bronchodilator (combined long-acting β2-agonist [LABA] and long-acting muscarinic antagonist [LAMA]). Because dual bronchodilators yield greater improvements in lung function and abatement of symptoms than a single bronchodilator,8,9 we used a dual bronchodilator to provide a rigorous test of whether bronchodilation benefits this patient population.
Methods
TRIAL OVERSIGHT
We conducted the Redefining Therapy in Early COPD (RETHINC) trial as an investigator-initiated, multicenter, blinded, randomized, controlled trial within the National Heart, Lung, and Blood Institute (NHLBI)–funded Pulmonary Trials Cooperative10 based on evidence generated from Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS).2 The trial was designed by the authors. The University of Pittsburgh Network Management Core and the leadership committee of the Pulmonary Trials Cooperative contributed to the writing of the protocol, operations, and site management. The 20 enrolling centers included academic, Veterans Affairs, and community medical centers. The NHLBI organized the data and safety monitoring board, and Novartis Pharmaceuticals donated masked trial medication and a placebo. Industry funders were not involved in the design or conduct of the trial, the collection or analysis of the data, the writing of the manuscript, or the decision to submit the report for publication. The protocol was approved by the institutional review boards at the University of Michigan and each participating center. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org.
PARTICIPANTS
We enrolled persons 40 to 80 years of age who currently or formerly smoked cigarettes with at least a 10-pack-year history, had respiratory symptoms as defined by a CAT score of 10 or higher, and had a FEV1:FVC of at least 0.70 and an FVC that was at least 70% of the predicted value after bronchodilator use. We excluded persons who had a primary diagnosis of asthma based on criteria from National Institute for Health and Care Excellence guidelines,11 those with other known concomitant lung disease, and those already using maintenance inhaled LAMA, LABA, fixed combination of LABA with an inhaled glucocorticoid, a short-acting anticholinergic agent, or fixed combination of a short-acting β2-agonist (SABA) and short-acting anticholinergic, unless the potential participant was able to undergo a 30-day medication washout. SABAs were allowed as needed during the trial, without specific instructions given regarding their use. A complete list of the inclusion and exclusion criteria is provided in the protocol. Recruitment methods included advertising in clinics, community venues, and social media. Some participants also participated in the SPIROMICS and COPDGene observational studies. All participants provided written informed consent.
TRIAL PROCEDURES
We used permuted block randomization with varying block sizes of 2, 4, and 6, stratified according to center, smoking status (current or former), and whether a medication washout was warranted. We randomly assigned participants in a 1:1 ratio to receive indacaterol (27.5 μg) plus glycopyrrolate (15.6 μg) or placebo twice daily for 12 weeks; these doses of indacaterol and glycopyrrolate are the Food and Drug Administration (FDA)–approved doses for the treatment of COPD in the United States, although they are lower than the doses approved elsewhere (110 μg of indacaterol and 50 μg of glycopyrrolate in Canada and 85 μg and 43 μg, respectively, in Europe). We planned to enroll 290 participants in each group. We administered the St. George’s Respiratory Questionnaire (SGRQ), CAT, and Baseline Dyspnea Index (BDI) at baseline and the Transition Dyspnea Index (TDI) at follow-up; performed spirometry (with Hankinson reference equations12) at baseline and 12 weeks; and followed up by telephone at 4 weeks to assess adverse events. SGRQ scores range from 0 to 100, with higher scores indicating worse health status; the minimum clinically important difference is 4 points. The minimum clinically important difference for the CAT score is 2 points. BDI scores range from 0 to 12, with higher scores indicating greater dyspnea at baseline. TDI scores are a measure of the change in dyspnea severity from the baseline value established by the BDI score; scores range from −9 to 9, with higher scores indicating greater decreases in dyspnea severity; the minimum clinically important difference is 1 point.
OUTCOMES
The primary outcome was a decrease (i.e., improvement) by more than 4 points in the SGRQ score after 12 weeks without treatment failure.13 We defined treatment failure as an increase in lower respiratory symptoms leading to treatment with a long-acting bronchodilator, glucocorticoid, or antibiotic agent. Important pre-specified secondary outcomes included a decrease by at least 2 points in the CAT score,14 a TDI score of at least 1,15, and a decrease by at least 4 points in the SGRQ score plus a TDI score of at least 1, all without treatment failure; the mean changes from baseline in the SGRQ and CAT scores; the TDI score; the change from baseline in predose FEV1 and inspiratory capacity measured 12 hours after receipt of treatment or placebo; the FEV1 assessed hourly over the first 3 hours after a dose of treatment or placebo (expressed as the area under the curve [AUC0–3hr]) at 12 weeks; treatment failure itself (as defined above); and the percentage of days with symptoms or use of rescue medication determined on the basis of information recorded by participants in a daily diary. Because of the coronavirus disease 2019 (Covid-19) pandemic, we evaluated the primary outcome by telephone in 20 participants in the treatment group and 22 in the placebo group. SGRQ administration by telephone has been shown to have good comparability to in-person administration.16
STATISTICAL ANALYSIS
We designed the trial to enroll 580 participants, which would provide 90% power to detect a 14-percentage-point difference in the percentage of participants meeting the primary outcome, accounting for 10% attrition (two-sided chi-square test), on the basis of data from the Novartis FDA development program studies.17 Because of the pandemic and time limits associated with the funding and drug supply, the trial ended with 535 participants having undergone randomization, with an estimated 87% power accounting for 10% attrition.
Disruption of our ability to conduct in-person trial visits during the Covid-19 pandemic led to missing or very delayed (>16 weeks after randomization) primary outcome data for 10 participants. Dropouts due to treatment failure were considered to be informative and contributed to the composite primary outcome. We considered other missing data to be missing completely at random. To handle this, we excluded participants who had neither treatment failure nor week 12 data for the primary outcome from the intention-to-treat population, yielding a modified intention-to-treat population for our primary analysis.
In addition, we performed four sensitivity analyses: one in which we reassigned 12 participants who were determined to have received the incorrect drug kit (i.e., not the one that had been randomly assigned), one in which we excluded 11 participants who had a FEV1:FVC of less than 0.70, one in which we limited our analyses to participants who completed the trial before the start of the Covid-19 pandemic, and one in which we included the 10 participants who were excluded from the modified intention-to-treat population because they had the primary outcome measured more 16 weeks after randomization. Finally, we analyzed a per-protocol population that excluded participants who had a protocol deviation or had unknown or incorrect adherence (defined as having taken <80% or >120% of protocol-specified doses).
The primary analysis was conducted by the University of Michigan Statistical Analysis of Biomedical and Educational Research (SABER) Group. Primary and secondary analyses with binary outcomes were conducted with the use of generalized-estimating-equation regression with logit link, with adjustment for the clinical center of recruitment, baseline smoking status, previous maintenance treatment warranting washout, body-mass index (BMI, the weight in kilograms divided by the square of the height in meters), and the baseline value of the outcome being evaluated. For continuous outcomes, we used linear mixed-effects models with adjustments for the same covariates. No interim statistical analysis of efficacy was conducted.
Prespecified subgroup analyses of the primary outcome were based on participant-reported baseline smoking status, baseline bronchodilator responsiveness,18,19, and BMI (>30 or ≤30). Additional exploratory subgroup analyses included subgroups defined according to sex, age (<65 years or ≥65 years), baseline percent of predicted FEV1 (either less than the median or greater than or equal to the median for all participants who underwent randomization), baseline inspiratory capacity (either less than the median or greater than or equal to the median for all participants who underwent randomization), and status with respect to chronic bronchitis according to the Medical Research Council (MRC) definition (cough and sputum on most days during at least 3 consecutive months for more than 2 successive years).20 We tested interactions between each of these subgroups and treatments. For secondary analyses, we provide 95% confidence intervals that have not been adjusted for multiplicity and are excluded from formal hypothesis testing.
Results
CHARACTERISTICS OF THE PARTICIPANTS
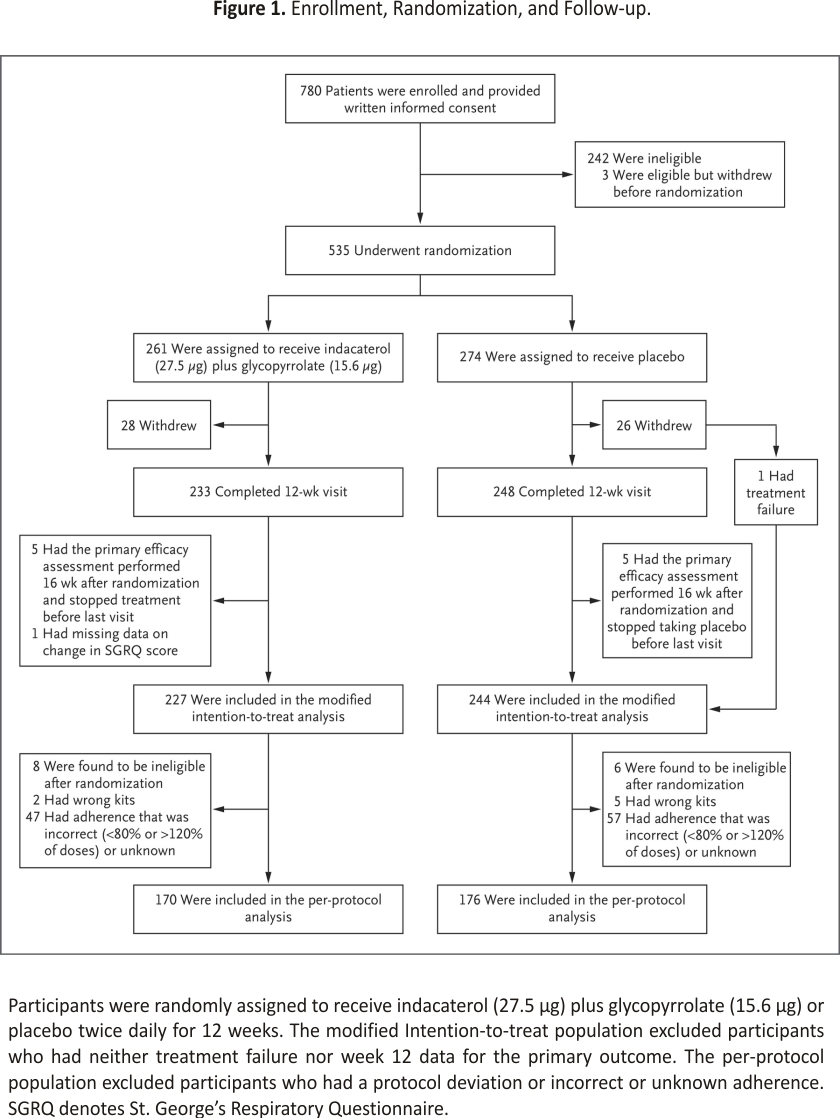
From July 2017 through March 2021, a total of 535 participants at 20 centers underwent randomization; 261 were assigned to receive active treatment and 274 to receive a placebo (Figure 1). Overall, 28% of participants were already using some type of inhaled COPD medication (SABA, short-acting muscarinic agonist [SAMA], LABA, LAMA, or inhaled glucocorticoid). Of the 32 potential participants who were screened but were already using long-acting bronchodilators and at least began washout, 20 underwent randomization, 5 were ultimately deemed ineligible, 2 were lost to follow-up, 2 were unable to undergo randomization because of the pandemic, and only 3 were not able to complete the washout. Overall, 4% of participants were enrolled after a washout of a maintenance COPD medication. After the exclusion of participants who did not complete the trial (53), had a missing SGRQ score (1), or had a very delayed final assessment because of the pandemic (10), a total of 471 participants were included in the modified intention-to-treat analysis. Participants who were excluded from the modified intention-to-treat analysis were slightly younger and more likely to be currently smoking tobacco and had slightly higher oxygen saturation than those who were included (Table S1 in the Supplementary Appendix, available at NEJM.org). Further excluding the 125 participants who had a major protocol deviation (defined as eligibility criteria violations for which no exemption was granted, nonadherence to treatment or placebo, or receipt of a prohibited medication) yielded 346 participants for the per-protocol analysis.
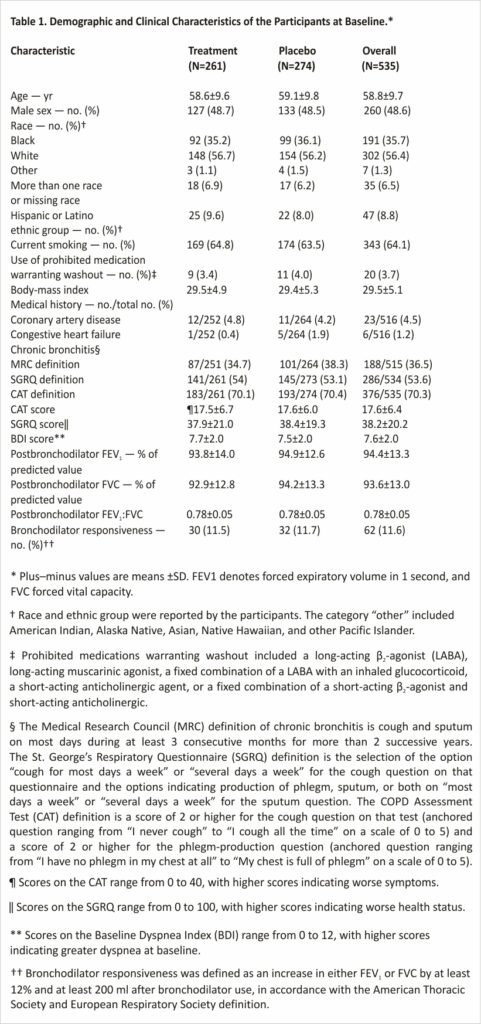
The baseline characteristics of the participants in the two groups were balanced in the intention-to-treat population (Table 1 and Table S2). Approximately half the trial population identified as female, 56.4% as White, and 35.7% as Black; 64.1% of the participants were currently smoking. The most common coexisting condition was diabetes (16.7%). Chronic bronchitis based on the MRC definition was present in 36.5% of the participants,20 although the percentage was higher when alternative definitions of chronic bronchitis based on the SGRQ and CAT were used.21,22 Baseline characteristics in the modified intention-to-treat population were also well balanced (Table S3); the balance between numbers of male and female participants was similar to that in the population in SPIROMICS, an observational study involving persons with a history of smoking. The prevalence of Black participants was higher and the prevalence of Hispanic or Latino participants lower than that observed in the 2020 U.S. Census; current epidemiologic data on this patient population are insufficient to determine whether these differences are expected (Table S4).
FOLLOW-UP AND OUTCOMES
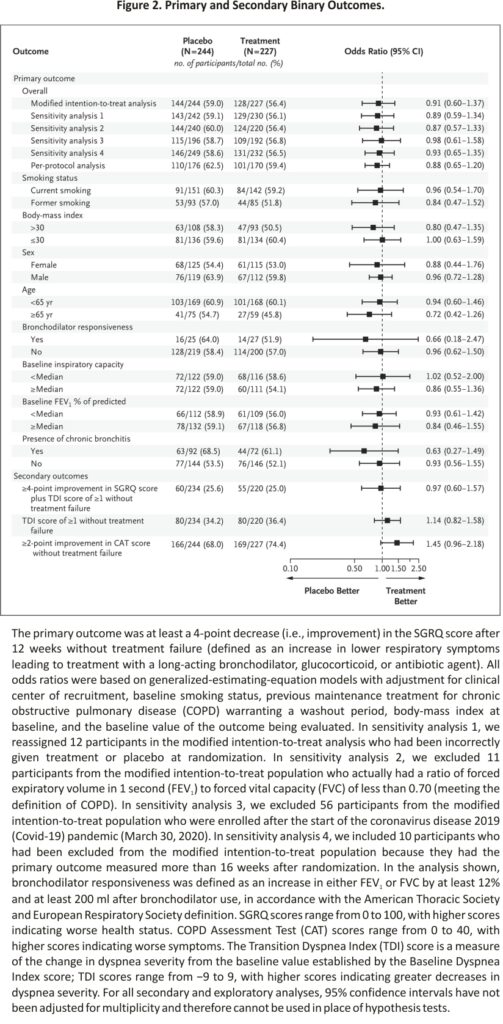
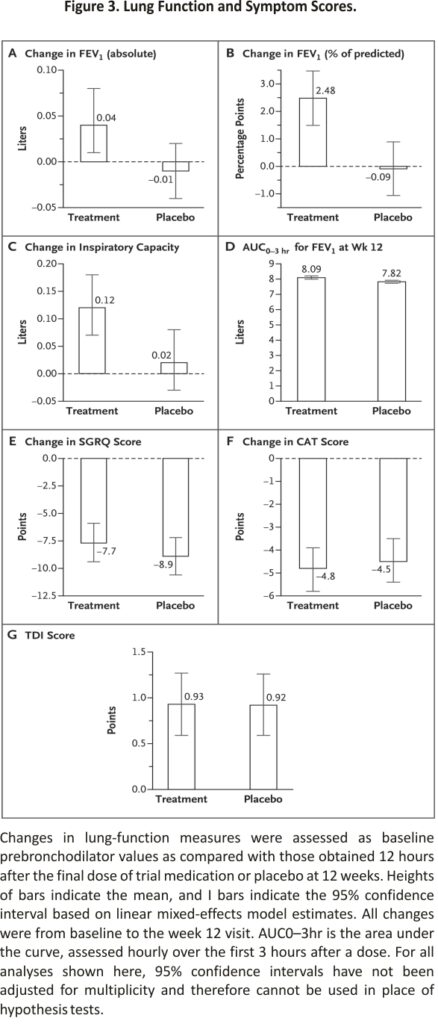
In our primary analysis involving the modified intention-to-treat population, we found no observable treatment effect; 128 of 227 participants (56.4%) in the treatment group and 144 of 244 participants (59.0%) in the placebo group had at least a 4-point decrease (improvement) in the SGRQ score without treatment failure (difference, −2.6 percentage points; 95% confidence interval [CI], −11.6 to 6.3; adjusted odds ratio, 0.91; 95% CI, 0.60 to 1.37; P=0.65). No significant treatment effect was found in any of our sensitivity analyses (Figure 2). The results were similar in the per-protocol analysis, with 101 of 170 participants (59.4%) in the treatment group and 110 of 176 (62.5%) in the placebo group having at least a 4-point decrease in the SGRQ score without treatment failure (odds ratio, 0.88; 95% CI, 0.65 to 1.20) (Figure 2). Treatment failure was uncommon, occurring in only 5 participants (2.2%) in the treatment group and 9 (3.7%) in the placebo group. Data on prespecified secondary analyses are provided in Figures Figure 2 and Figure 3 and Table 2. The results of prespecified subgroup analyses of the primary outcome are shown in Figure 2.
ADHERENCE AND SAFETY
Adherence was high, with 88% of doses taken in both the treatment group and the placebo group. Four serious adverse events occurred in the treatment group (in 4 participants [1.5%]), and 11 occurred in the placebo group (in 8 participants [2.9%]), with no deaths and no events deemed by the investigators to be potentially related to treatment or placebo. The most common nonserious adverse events were cough (3.4% of participants in the treatment group and 4.4% in the placebo group) and headache (3.4% and 4.4%, respectively) (Table S5).
Discussion
We found that dual long-acting bronchodilator treatment did not decrease respiratory symptoms in persons who currently or formerly smoked cigarettes and had substantial respiratory symptoms despite also having preserved lung function as assessed by spirometry. This stands in contrast to data on symptom abatement with dual long-acting bronchodilators in tobacco-exposed persons who meet the criteria for COPD.17
In the absence of clinical trial data, physicians have responded to this patient population in the “real world” by prescribing treatments known to work for COPD or asthma. In SPIROMICS, we found that 43% of these patients used bronchodilators: 31% used SABAs, 11% SAMAs, 31% LAMAs, 15% LABAs, and 23% inhaled glucocorticoids.2 In the COPDGene study, 20% of patients with normal lung function on spirometry who had one or more impairments (increased respiratory symptoms, history of severe exacerbation, CT abnormality, or reduced 6-minute walk distance) used respiratory medication.4 This is not surprising. Spirometry is underused in primary care,6,7, and either the diagnosis of COPD is incorrectly assumed without spirometry or, despite a lack of clinical trial data in this population, treatments found to be beneficial in COPD have been extended to this population.
Our results have important implications for clinical practice. Preserved lung function on spirometry (FEV1:FVC ≥0.70) in a person with current or former exposure to smoked tobacco and respiratory symptoms should generally discourage the prescription of bronchodilators for symptom control. This stands in contrast to the use of bronchodilators in patients with diagnoses of bona fide COPD who, on average, do derive symptomatic benefits from this treatment.17 It follows that it is important to distinguish between the two groups of patients, which is often not done in clinical practice, in which the use of spirometry in primary care for COPD has historically been infrequent.6 It is important to note, however, that we did not study inhaled glucocorticoids, azithromycin, or other COPD medications or therapeutics that target pathologic mucus.
Our trial has some limitations. Symptoms in some of our participants may have been driven by factors other than pulmonary abnormalities — for example, cardiac disease or sleep apnea — and a more narrowly defined patient population might have benefitted. For instance, the trial may have been underpowered to study the subgroup of participants with chronic bronchitis specifically. Furthermore, we enrolled only a small sample of participants who were taking long-acting bronchodilators before enrollment; it is possible that patients identified by their physicians as needing these medications are a unique subgroup that benefits from treatment. We observed abatement of symptoms in both the treatment group and the placebo group, which suggests a strong placebo effect (improvement directly related to receipt of any type of therapy), a Hawthorne effect (improvement related to being in a clinical trial), or regression to the mean (reduction in symptoms after selection for substantial symptoms). Given that we recruited participants with substantial respiratory symptoms and used a symptom score as our primary outcome, we suspect that regression to the mean contributed to the observed improvements (under-scoring the value of the placebo control). We know that persons with a history of smoking who have symptoms despite having preserved lung function on spirometry are at increased risk for respiratory exacerbations, and our follow-up period was too short to adequately assess the effects of treatment on exacerbations. It is also possible that 12 weeks was not a long enough period to observe symptomatic improvement; however, given the significant improvement in SGRQ score that has been documented at 12 weeks with the use of indacaterol plus glycopyrrolate in two COPD clinical trials,17 we believe this is less likely. Finally, although we used the drug doses that are FDA-approved for COPD in the United States, the higher doses that are approved in other countries might produce different results.
Our trial provides data on the treatment of patients without COPD who have a history of smoking tobacco and who have respiratory symptoms despite having preserved lung function on spirometry. The number of such persons is likely to be substantial. Data from the general population cohort study CanCOLD suggest that 25% of persons with any smoking history and normal lung function on spirometry report substantial dyspnea on exertion.23 Smoking-cessation therapy remains a primary goal for this patient population. However, our data suggest that long-acting bronchodilators do not result in the abatement of respiratory symptoms in these patients. Further research is urgently needed to better understand and treat the respiratory disease in these persons.
Supported by grants from the National Heart, Lung, and Blood Institute (HL128952, HL128954, HL137880, K24HL138188, K24HL137013, and K24HL140108) and the National Center for Advancing Translational Sciences (KL2TR001882) and by Sunovion Pharmaceuticals (financial support to the clinical centers for costs of conducting the trial through the COPD Foundation). Novartis Pharmaceuticals donated the trial medication and placebo.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Health Care System. The work here represents the views of the authors and not necessarily the views of the Department of Veterans Affairs or the U.S. government.
Author Affiliations
From the Division of Pulmonary and Critical Care (M.K.H., C. Meldrum) and the School of Public Health (W.Y., D.W., E.W.), University of Michigan, Ann Arbor; the Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine (M.A., S.C.L., P.G.W.) and the Cardiovascular Research Institute (S.C.L., P.G.W.), University of California San Francisco, and the San Francisco Veterans Affairs (VA) Healthcare System (M.A.) — both in San Francisco; the Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at UCLA (I.Z.B., R.G.B., C.B.C.), and the Lundquist Institute for Biomedical Innovation at Harbor– UCLA Medical Center (W.W.S.) — both in Los Angeles; the Division of Pulmonary and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (S.-A.B., N.N.H., R.A.W.); the Division of Pulmonary, Critical Care, and Occupational Medicine, University of Iowa, Iowa City (A.P.C.); the Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University, Philadelphia (G.J.C.); the Division of Pulmonary, Allergy, and Critical Care Medicine, Heersink School of Medicine, University of Alabama at Birmingham, Birmingham (M.T.D.); Geisel School of Medicine at Dartmouth and Pulmonary and Critical Care Medicine, VA Medical Center, White River Junction, VT (F.D.); the Division of Pulmonary, Critical Care, and Sleep Medicine, Houston Methodist Academic Medicine Associates, Houston (R.J.F.); the Division of Pulmonary and Critical Care Medicine, Northwestern University Feinberg School of Medicine (R.K.), and the Breathe Chicago Center, Division of Pulmonary, Critical Care, Sleep, and Allergy, University of Illinois Chicago (J.A.K.) — both in Chicago; the Department of Genetic Medicine (R.J.K.) and Joan and Sanford I. Weill Department of Medicine (R.J.K., F.J.M.), Weill Cornell Medicine and New York–Presbyterian Hospital, and the Division of Pulmonary, Critical Care, and Sleep Medicine, Icahn School of Medicine at Mount Sinai (L.R.) — both in New York; the Division of Respiratory, Critical Care, and Occupational Pulmonary Medicine, University of Utah School of Medicine, Salt Lake City (R.E.K.); East Carolina University, Greenville (V.M.), and Duke University School of Medicine, Durham (A.M.) — both in North Carolina; HealthPartners Institute, Bloomington (C. McEvoy), and Minneapolis VA Healthcare System, Minneapolis (C.H.W.) — both in Minnesota; and the Division of Pulmonary, Allergy, and Critical Care Medicine (T.N., F.C.S.) and Epidemiology Data Center (S.R.W.), University of Pittsburgh, Pittsburgh.
Dr. Han can be contacted at mrking@umich.edu or at the Division of Pulmonary and Critical Care, University of Michigan, 1150W. Medical Center Dr., 6220 MSRB III SPC 5642, Ann Arbor, MI 48109-5642.
The members of the RETHINC Study Group are listed in the Supplementary Appendix, available at NEJM.org.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017;195:557-582.
2. Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016;374: 1811-1821.
3. Kesimer M, Ford AA, Ceppe A, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017;377:911-922.
4. Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015;175:1539-1549.
5. Tan WC, Bourbeau J, Hernandez P, et al. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax 2014;69:7 09-717.
6. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest 2007;132: 403-409.
7. Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med 2018;142:48-52.
8. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013;42:1484-1494.
9. Singh D, Donohue JF, Boucot IH, Barnes NC, Compton C, Martinez FJ. Future concepts in bronchodilation for COPD: dual- versus monotherapy. Eur Respir Rev 2021;30:210023-210023.
10. Han MK, Ye W, Kim D-Y, Woodruff P. Design of the redefining therapy in early COPD study. Chronic Obstr Pulm Dis 2020;7:382-389.
11. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. February 25, 2004 (https://www.nice.org.uk/guidance/cg12. opens in new tab).
12. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159: 179-187.
13. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2: 75-79.
14. Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014;2:195-203.
15. Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005;2:99-103.
16. Rocha V, Jácome C, Martins V, Marques A. Are in-person and telephone interviews equivalent modes of administrating the CAT, the FACIT-FS, and the SGRQ in people with COPD? Front Rehabilit Sci 2021;2:729190-729190 (https://www.frontiersin. org/articles/10.3389/fresc.2021.729190/full. opens in new tab).
17. Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1, and FLIGHT2: efficacy and safety of QVA149 (indacaterol/ glycopyrrolate) versus its monocomponent and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:1068-1079.
18. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-338.
19. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26: 948-968.
20. Definition and classification of chronic bronchitis for clinical and epidemiological purposes: a report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965;1: 775-779.
21. Stott-Miller M, Müllerová H, Miller B, et al. Defining chronic mucus hypersecretion using the CAT in the SPIROMICS cohort. Int J Chron Obstruct Pulmon Dis 2020;15: 2467-2476.
22. Choi JY, Yoon HK, Shin K-C, et al. CAT score and SGRQ definitions of chronic bronchitis as an alternative to the classical definition. Int J Chron Obstruct Pulmon Dis 2019; 14:3043-3052.
23. Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax 2015; 70:822-829.
Credits: Han MK, Ye W, Wang D, White E, Arjomandi M, Barjaktarevic IZ, Brown SA, Buhr RG, Comellas AP, Cooper CB, Criner GJ, Dransfield MT, Drescher F, Folz RJ, Hansel NN, Kalhan R, Kaner RJ, Kanner RE, Krishnan JA, Lazarus SC, Maddipati V, Martinez FJ, Mathews A, Meldrum C, McEvoy C, Nyunoya T, Rogers L, Stringer WW, Wendt CH, Wise RA, Wisniewski SR, Sciurba FC, Woodruff PG; RETHINC Study Group. Bronchodilators in Tobacco-Exposed Persons with Symptoms and Preserved Lung Function. N Engl J Med. 2022 Sep 29;387(13):1173-1184. doi: 10.1056/NEJMoa2204752. Epub 2022 Sep 4. PMID: 36066078.