Artak Labadzhyan, Kristopher Wentzel, Omid Hamid, Kamlynn Chow, Sungjin Kim, Lawrence Piro, Shlomo Melmed
Abstract
Context
Incidence and awareness of endocrine-related adverse events (ERAE) associated with the use of immune checkpoint inhibitors (ICI) have grown with increased ICI use, yet mechanisms for ERAE prediction, surveillance, and development are not well established.
Objective
We prospectively evaluated the impact of endocrine autoimmunity on ERAE development and overall survival (OS).
Methods
Adults ≥ 18 years of age prescribed ICI treatment for advanced or metastatic solid tumors and no known active/ past endocrine disorders were eligible for enrollment. Thyroid, adrenal, and pancreatic antibodies as well as hormone levels were assessed prior to ICI treatment and at 8 to 9 weeks and 36 weeks after treatment for ERAE in relation to the presence and changes in endocrine-specific antibodies, hormone levels, and OS.
Results
Sixty patients were enrolled and ERAE was detected in 14 (23.3%), with a median onset of 52 days (IQR, 38.5-71.5) after the first ICI dose. Hypothyroidism occurred in 12 (20%) patients, and 2 (3.33%) patients developed hypophysitis. Diabetes and primary adrenal insufficiency were not observed. Antibodies were detected in 14 patients (11 at baseline, 3 developed during follow-up) and their presence was significantly associated with ERAE (R2 59.3%, P < 0.001). Thyroid peroxidase antibody (20%) and thyroid-stimulating immunoglobulin (3.3%) were most common, and anti-GAD was present in 1 patient. The presence of ERAE was associated with a more favorable OS (P = 0.001).
Conclusion
Endocrine-specific autoantibodies play an important role in ERAE pathogenesis and may serve as predictive markers for early identification and treatment of ICI-induced endocrinopathies.
Keywords: immune checkpoint inhibitors, endocrine-related adverse events, autoantibodies
Immune checkpoint inhibitors (ICI) block cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death 1 (PD-1), or programmed death ligand-1 (PD-L1), enabling activation of the immune system to target cancer cells 1,2. Immune-related adverse events (irAEs) are seen in 10% to 60% of patients treated with ICI, with a significant risk for grade 3/4 irAEs 3. There is a current knowledge gap in relation to predictive markers for these irAEs.
The incidence and awareness specifically of endocrine-related adverse events (ERAE) have grown with increased ICI use, including hypophysitis, primary thyroid disease, adrenal insufficiency, and insulin-dependent diabetes mellitus 4,5. Hypophysitis, characterized by chronic or subacute pituitary inflammation, is associated mostly with anti-CTLA-4 therapy used alone or in combination with anti-PD1 therapy, with a reported incidence of 3% to 7% in treated patients. By contrast, thyroiditis and hypothyroidism are the most commonly reported ERAE in patients treated with anti-PD-1/PD-L1 ICI, with an incidence ranging from 3% to 13% 5. Older age, male sex, and duration of treatment may be risk factors for developing ERAE 6-10.
Mechanisms for ERAE development are not well established, although the presence of preexisting disordered autoimmunity likely plays a key role 11. Indeed, several endocrine-specific antibodies have been linked to ICI-induced ERAE. For example, the presence of anti-thyroid antibodies may be a risk factor for developing thyroiditis or hypothyroidism 12, while the adrenal antibody, anti-21-hydroxylase (21-OH), has been reported in the rare ERAE ICI-induced autoimmune polyendocrine syndrome type 2 13,14. Autoimmune diabetes-related antibodies such as anti-glutamic acid decarboxylase (GAD) antibodies are positive in 50% of patients who develop ICI-induced insulin-dependent diabetes mellitus, another rare ERAE 15,16.
Prospective evidence regarding the role of endocrine autoimmunity in patients treated with ICI is lacking. We therefore prospectively studied ERAE development in patients treated with anti-CTLA-4, anti-PD1, and/or anti-PD-L1 ICI for advanced or metastatic cancer. We investigated endocrine-specific autoantibodies and pituitary hormone levels at treatment initiation and subsequently as predictors of ERAE development, and we measured the impact of ERAE development on OS in patients treated with ICI.
Methods
Study Approval
The study protocol was approved by the Cedars-Sinai Institutional Review Board (IRB #PRO00045026). All patients provided written informed consent prior to participation. The trial was conducted in accordance with the principles of the Declaration of Helsinki.
This prospective study evaluated adult patients ≥ 18 years of age treated with ICI for advanced or metastatic solid tumors between 2018 and 2021. Patients with advanced or metastatic breast cancer, melanoma, non-small cell lung cancer (NSCLC), ovarian cancer, prostate cancer, renal cell carcinoma, or uterine cancer who were prescribed de novo treatment with anti-CTLA-4 (ipilimumab), anti-PD-1 (cemiplimab, nivolumab, pembrolizumab), or anti-PD-L1 (atezolizumab, durvalumab) were eligible for enrollment. Patients who had already started treatment as well as those with prior ICI use were not eligible.
Patients were excluded for active/past endocrine disorders, including pituitary disease of any kind, hypothyroidism, hyperthyroidism, hyperparathyroidism, adrenal insufficiency, Cushing syndrome, hypogonadism, or current/past hormone replacement. Past systemic corticosteroid use was acceptable if such treatment was discontinued more than 4 weeks prior to enrollment. Patients with a history of head and neck radiation or other active cancer treatment that could affect endocrine function, including the use of tyrosine kinase inhibitors, were also excluded.
Assessments
Baseline laboratory tests were collected on the day of or up to 7 days prior to the initial ICI infusion. Subsequent blood collections were obtained at weeks 8 to 9 and at week 36 after the first infusion, or at the time of an ERAE occurrence.
Autoantibodies tested included thyroid peroxidase antibody (TPO-Ab) (Inova Quanta Lite TPO, ELISA), thyroid-stimulating immunoglobulin (TSI) (Quest Diagnostics, in vitro bioassay), 21-OH antibody (Quest Diagnostics, ELISA), and GAD-65 antibody (Quest Diagnostics, ELISA). Hormone levels tested included insulin-like growth factor 1 (IGF-1) (Quest Diagnostics, LC/MS), thyroid-stimulating hormone (TSH) (Architect, chemiluminescent microparticle immunoassay [CMIA]), free thyroxine (FT4) (Architect, 2-step immunoassay [IA], CMIA), total T3 (IA), adrenocorticotrophic hormone (ACTH), (Quest Diagnostics, IA), cortisol (Architect, quantitative IA, CMIA), prolactin (Architect, 2-step IA, CMIA), follicle-stimulating hormone (FSH) (2-step IA, CMIA), luteinizing hormone (LH) (2-step IA, CMIA), estradiol (Architect, IA, CMIA), and testosterone (Quest Diagnostics, LC/MS).
ERAE was defined by the post-treatment development of biochemical abnormalities that met accepted criteria for diagnosing endocrine disease 17-20. Specifically, primary hypothyroidism was defined as TSH > 10 mIU/L or TSH above the upper limit of normal and FT4 below the lower limit of normal. Adrenal insufficiency was defined as a morning cortisol level < 3 µg/dL with symptoms, or a cortisol level < 18 µg/dL on a cosyntropin stimulation test. Hypogonadism was defined as morning gonadal hormones (FSH, LH, estradiol for women, testosterone for men) below the lower end of the reference range for age or menopausal status. Diabetes was diagnosed if fasting blood glucose was ≥ 126 mg/ dL and/or hemoglobin A1C was ≥ 6.5%. Hypopituitarism was defined as the presence of one or more pituitary or central hormonal deficiency: adrenal insufficiency and ACTH < 10 pg/mL; low FT4 with TSH below/within the lower end of reference range; or hypogonadism and FSH or LH below/within the lower end of the reference range. GH deficiency was defined as at least 2 other pituitary hormone deficiencies and low IGF-1 for age. Hypophysitis was defined as one or more pituitary hormone deficiencies and evidence of pituitary gland or stalk enlargement on the brain or pituitary MRI. If MRI was not available, hypophysitis was defined based on headache, vision changes, nausea, and hyponatremia in the setting of hypopituitarism.
Statistical Analysis
Data were presented as frequency (percentage) for categorical variables and as mean (±SD) and median (interquartile range [IQR]) for continuous variables. Univariate associations between ERAE and biomarkers of interest at baseline and change from baseline to week 8/9 or week 36 were examined with Fisher’s exact test, analysis of variance (ANOVA), or Wilcoxon rank-sum test, as appropriate. A logistic regression model was employed to identify variables associated with ERAE with and without adjustment for potential confounding factors. Overall survival (OS) was calculated from treatment start to the date of death or censored at the last follow-up. Survival functions were estimated by the Kaplan-Meier method and the log-rank test. All statistical tests were 2-sided and a P value of < 0.05 was considered statistically significant. Analyses were performed using JMP, Version 15 (SAS Institute Inc., Cary, NC).
Results
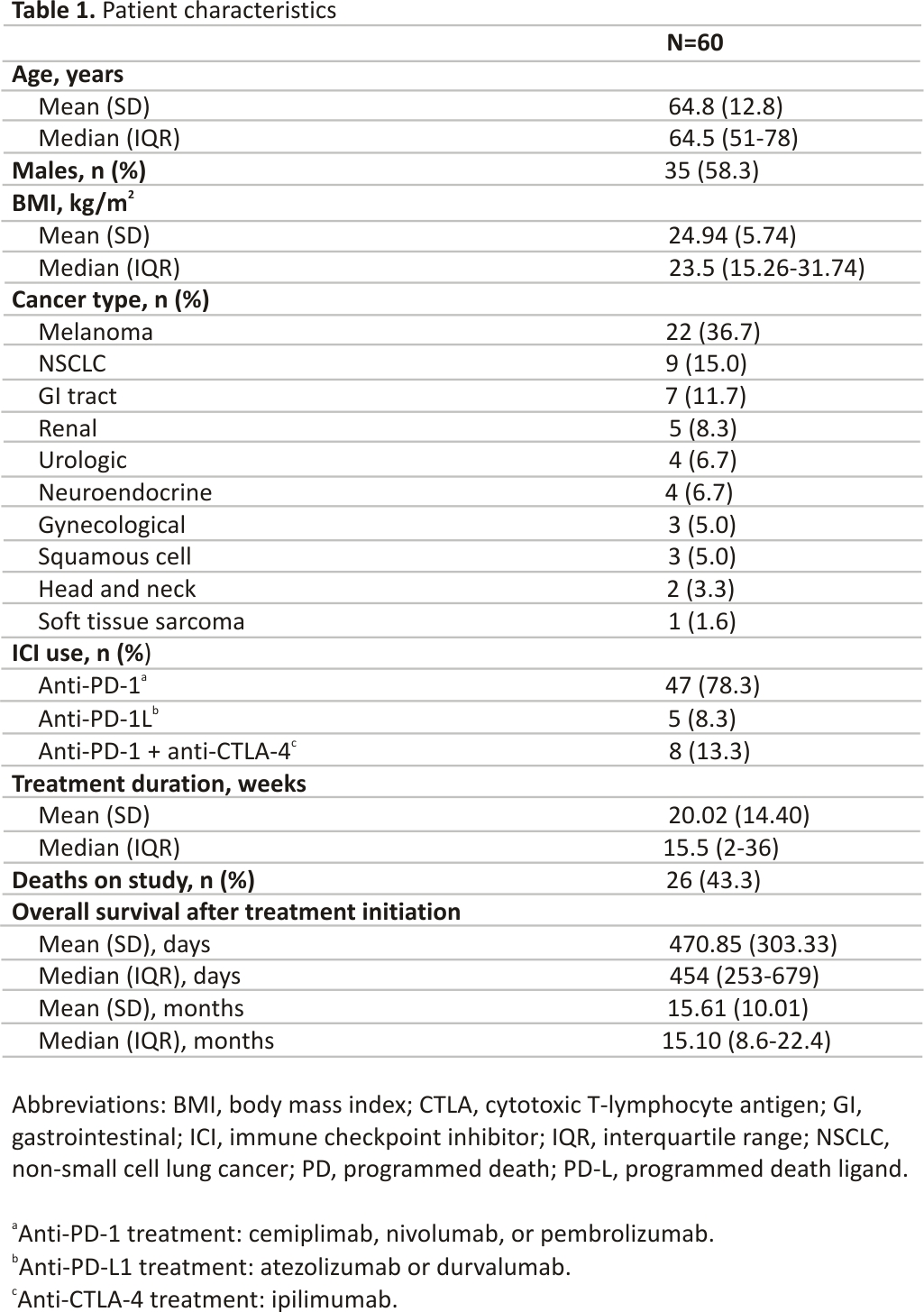
Sixty patients were enrolled (Table 1). The mean age was 64.8 (±12.8) years. The majority of patients were male (n = 35, 58.3%). Melanoma was the most common cancer type (n = 22, 36.7%), followed by NSCLC (n = 9, 15%) and gastrointestinal tract (n = 7, 11.7%), and renal (n = 5, 8.3%) cancers. The majority of patients (n = 47, 78.3%) were treated with anti-PD-1 therapy (cemiplimab, nivolumab, or pembrolizumab); 5 patients (8.3%) received anti-PD-L1 therapy (atezolizumab or durvalumab), and 8 patients (13.3%) received combination treatment with anti-CTLA-4 ipilimumab plus anti-PD-1 nivolumab. At 36 weeks, at the last, follow-up time point, 34 patients were alive (56.7%), with a mean OS of approximately 15.6 months after initiation of ICI therapy (Table 1).
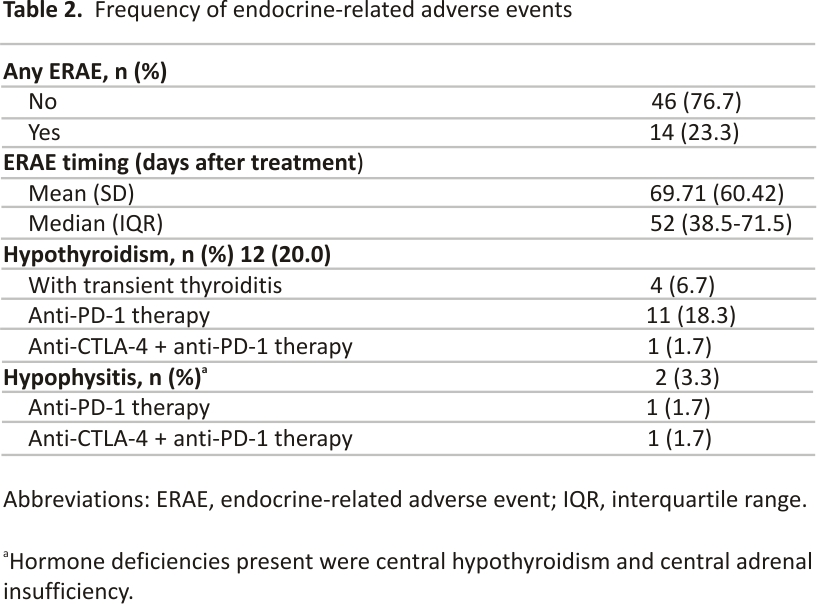
ERAE occurred in 14 (23.3%) patients, with a median onset of 52 days (IQR, 38.5-71.5) after the first dose of ICI (Table 2). Hypothyroidism with or without transient thyroiditis was the most common type of ERAE observed, occurring in 12 (20%) patients. Two patients (3.3%) developed hypophysitis with central hormone deficiencies, including central hypothyroidism (low TSH and free T4 levels) and central adrenal insufficiency (low ACTH and morning cortisol levels). Hormone deficiencies ascribed to hypothyroidism or hypophysitis persisted for the duration of the study. Of the 12 patients who developed hypothyroidism, 11 had received anti-PD-1 therapy alone, and 1 patient had received combination treatment with anti-CTLA-4 plus anti-PD-1. Of the 2 patients who developed hypophysitis, 1 had received anti-PD-1 therapy alone, and the other received combination treatment with anti-CTLA-4 plus anti-PD-1.
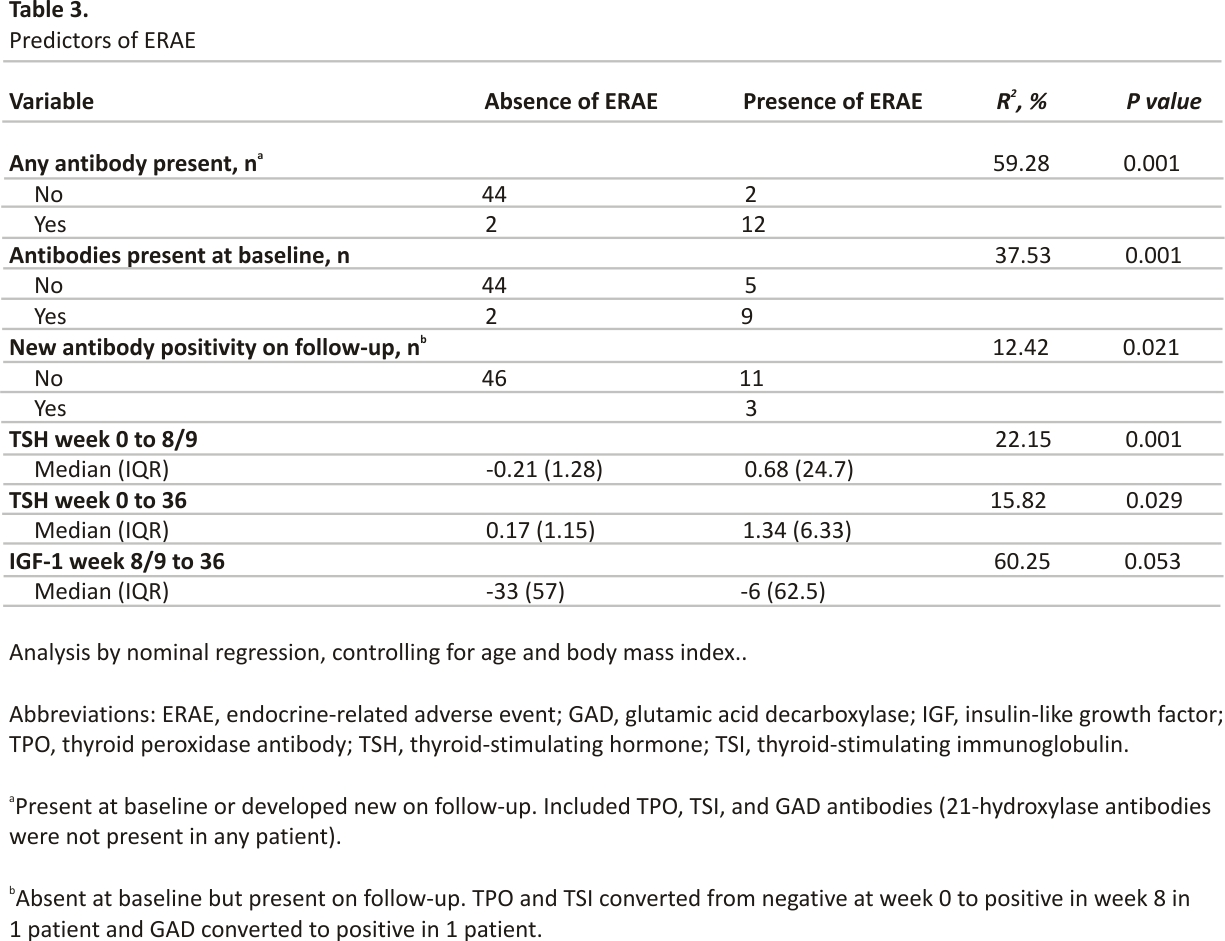
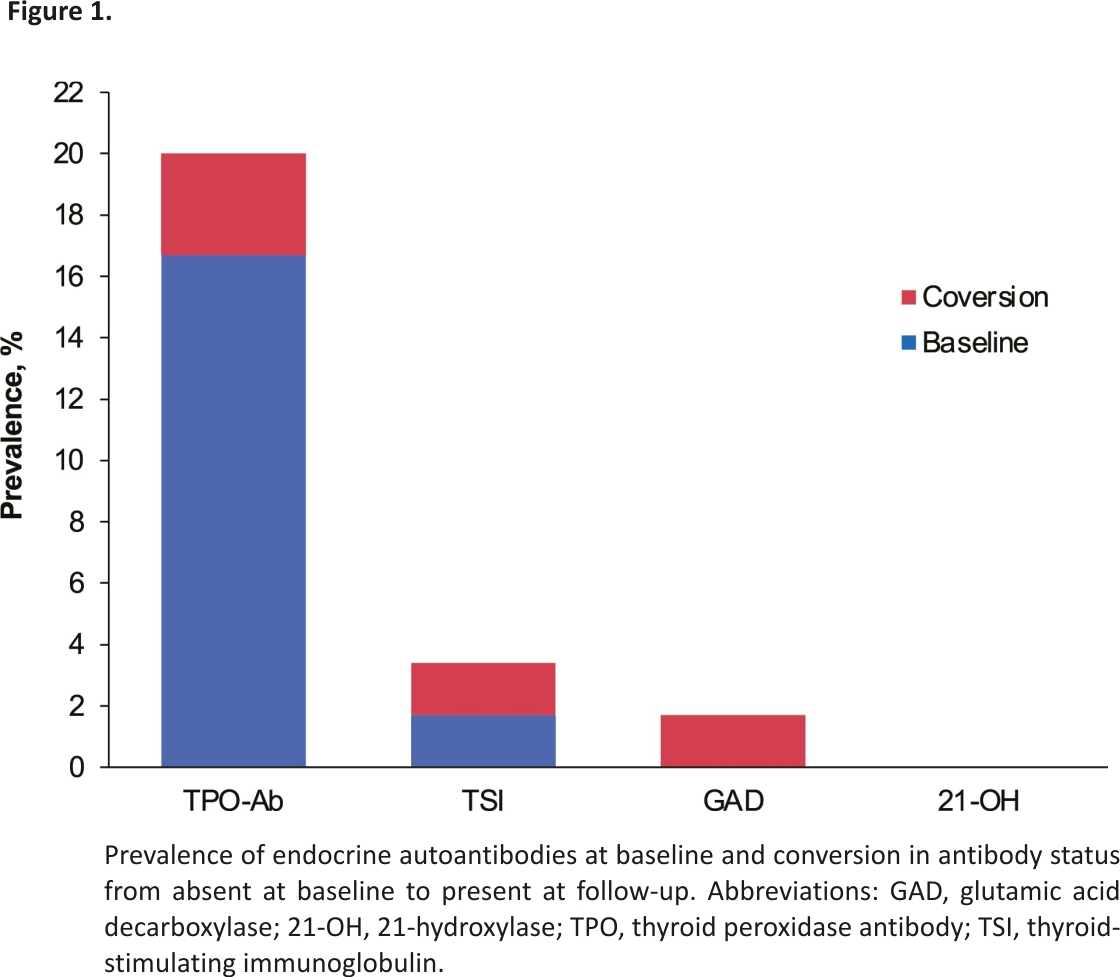
Of the 14 patients that developed ERAE, endocrine-specific antibodies were present at baseline in 9 patients (64.3%), 3 did not have antibodies at baseline but developed new antibodies on week 8/9 follow-up, and 2 did not have antibodies at baseline or follow-up assessments (R2 59.28%, P < 0.001). Two of the 46 patients who did not develop ERAE tested positive for antibodies. Overall, antibodies were detected in 14 patients, present either at baseline or newly developed during follow-up (Table 3). TPO-Ab was present in 12 (20%) patients and TSI in 2 (3.3%) patients; 1 patient had both TPO and TSI antibodies. Anti-GAD65 was present in 1 (1.7%) patient, and none had anti-21-OH (Fig. 1). Three patients converted to antibody positivity from absent at baseline to present at week 8/9 follow-up, and all 3 developed ERAE (R2 12.42%, P = 0.021) (Table 3). ERAE was noted at the same time as antibody conversion in 1 patient, and at 50 days (106 days from baseline) and 91 days (154 days from baseline) after antibody conversion in the other patients. In 1 patient, both TPO-Ab and TSI converted to positive at week 8/9; in the other, anti-GAD65 converted to positive at week 8/9.
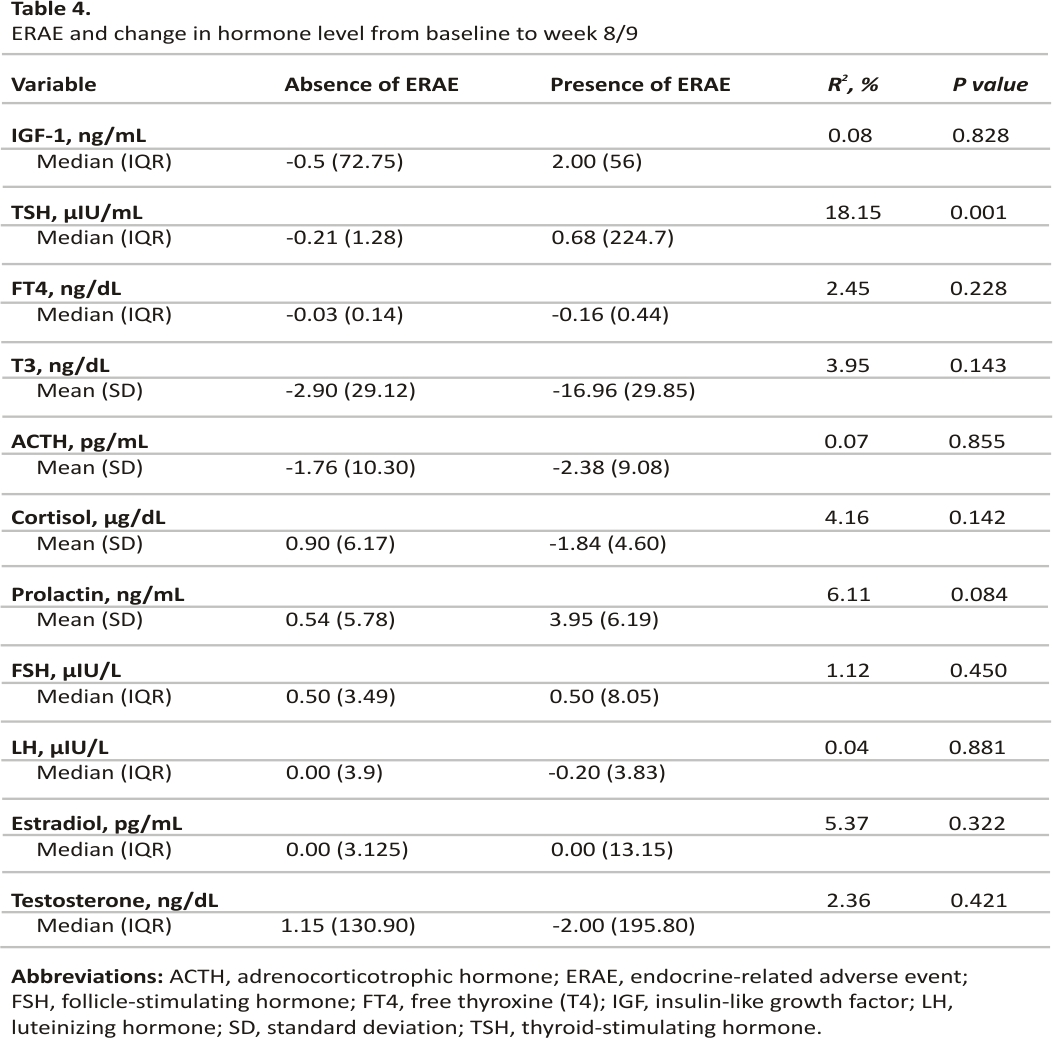
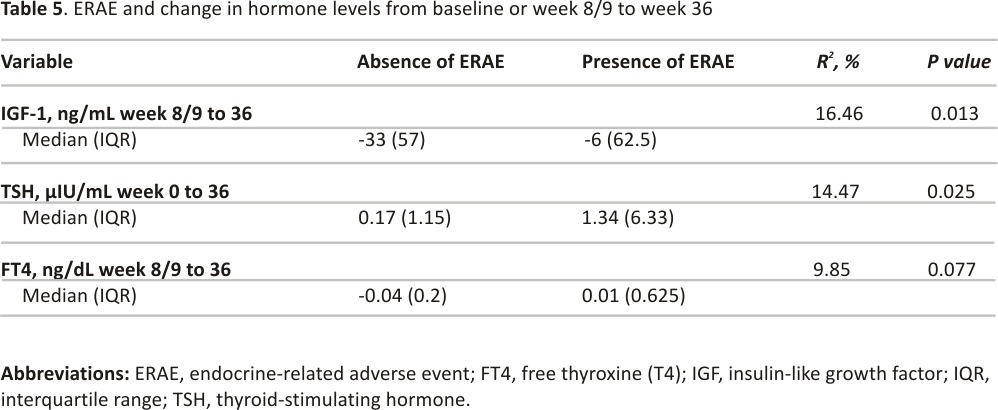
A decrease in TSH (median, −0.21 µIU/mL) from baseline to week 8/9 was associated with the development of ERAE (R2 18.15%, P = 0.001). No other hormone changes were significantly associated with ERAE during this follow-up period (Table 4). With longer follow-up, both an increase in TSH (median, 0.17 µIU/mL) from baseline to week 36 and a decrease in IGF-1 (median, −33 ng/mL) from week 8/9 to week 36 were associated with ERAE (Table 5). The decrease in FT4 from week 8/9 to week 36 was not associated with ERAE.
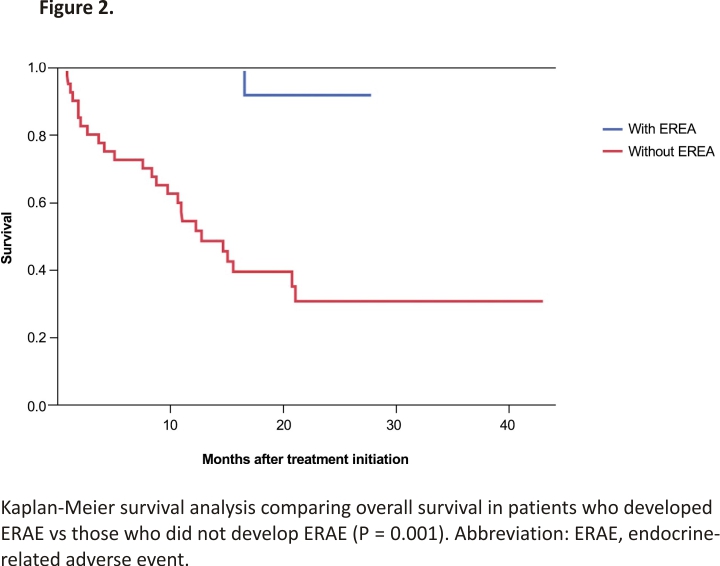
The presence of ERAE was associated with a more favorable OS (Fig. 2). Of the 14 patients who developed ERAE, 13 were alive at the end of the study (92.6%), with a mean OS of 16.6 months. By contrast, of the 46 patients who did not develop ERAE, 21 were alive at the end of the study (45.7%), with a mean OS of 14.8 months (P = 0.001).
Discussion
Endocrine-specific antibodies play an important role in the pathogenesis, diagnosis, and monitoring of endocrine disease 17,19,20, and the results of this study show that these antibodies are also important predictive factors for developing ERAE in patients receiving ICI for advanced or metastatic cancer. To our knowledge, prospective evaluation of the dynamics of multiple antibodies targeting different endocrine glands, including anti-TPO, TSI, GAD-65, and 21-OH, in patients who are ICI-naïve, has not been previously reported. The prospective design of this study allowed for a comprehensive laboratory evaluation of multiple hormonal axes at several time points and strengthened statistical analysis by allowing for standardization of laboratory assays, the timing of evaluation, and diagnostic criteria. Real-time monitoring of concomitant medication and other severe illnesses that may alter hormone levels also buttressed confidence in the accuracy of ERAE diagnosis and associated risk factors.
Of the 14 patients who had at least 1 endocrine-related antibody detected at baseline or during follow-up, 12 (85.7%) developed ERAE. Conversely, only 2 of 46 patients (4.3%) in the antibody-negative group developed ERAE. TPO-Ab was the most common antibody present at baseline, and nearly all anti-TPO positive patients (9/10, 90%) developed ERAE after initiation of ICI.
The strong associations between endocrine-specific antibodies and ERAE may provide clues to the underlying mechanism of ICI-induced endocrinopathies. Euthyroid autoimmune thyroid disease, in which TPO-Ab is present with normal thyroid function, is usually an indolent problem in the general population, with a 1% to 4% annual risk of progression to overt hypothyroidism 21. The presence of such endocrine antibodies may reflect a subclinical disease process, and immune system activation by ICI could enable the conversion of euthyroid autoimmune thyroid disease to overt hypothyroidism. Such a mechanism would be analogous to postpartum thyroiditis observed in euthyroid women with TPO-Ab, thought to be secondary to immunological rebound from the suppressed immune function of pregnancy 22,23.
The 2 patients who developed hypophysitis in our study had positive anti-TPO antibodies at baseline and both had multiple central hormone deficiencies, including central adrenal insufficiency. The presence of TPO-Ab, a marker of primary thyroid disease 24, may have indicated general endocrine autoimmunity in these patients, thus placing them at higher risk for developing hypophysitis. Such cross-reactivity of autoimmune risk predisposing to increased risk for hypophysitis has been reported in the setting of both endocrine and non-endocrine autoimmune diseases 11,25-27. In a systematic review of ICI-treated patients with preexisting autoimmune diseases, including rheumatological and autoimmune thyroid disease, hypophysitis was the second most common irAE, with an incidence of 5% 11. Accordingly, a propensity for autoimmunity likely plays at least a partial role in the risk for ERAE.
Other studies have pointed to a more specific autoantibody-mediated mechanism for the pathogenesis of hypophysitis. Serological analysis of recombinant cDNA expression (SEREX) identified anti-GNAL and anti-ITM2B autoantibodies as correlates of ICI-induced hypophysitis 28, while antibodies directed at TSH-secreting, FSH-secreting, and ACTH-secreting pituitary cells were detected in patients who developed ipilimumab-induced hypophysitis 29. These antibodies are of interest for future studies; however, they are currently not available for measurement in clinical laboratories and their utility in clinical practice remains unknown.
Despite compelling evidence supporting a role for autoantibodies in the pathogenesis of ERAE, the underlying mechanism is likely multifactorial, including humoral immunity as well as direct cytotoxicity. In mice, injection with anti-CTLA-4 led to infiltrating mononuclear cells in the pituitary gland and positive complement tissue staining, suggesting pituitary-specific inflammation 29. Pituitary adenomas express PD-L1 30, and similar expression in normal pituitary tissue may provide a target for cytotoxic pituitary damage from anti-PD-1/PD-L1 treatment.
Notably, 21-OH antibodies, a marker of primary adrenal insufficiency, were not detected either at baseline or throughout the study. The lack of observed adrenal autoimmunity may explain why ICI-induced primary adrenal insufficiency is so rare compared with hypophysitis or isolated central adrenal deficiency 31. Indeed, in a multicenter retrospective review, isolated central adrenal insufficiency was the sole pituitary hormone deficiency in 18 of 22 patients who developed hypophysitis secondary to anti-PD-1 therapy 32. Whether some percentage of central adrenal insufficiency cases may be due to a paraneoplastic syndrome that leads to anti-corticotroph autoantibodies should be considered 33.
None of the patients in our study developed diabetes and none showed anti-GAD65 antibodies at baseline. Interestingly, one patient converted to anti-GAD positive at the 8/9 week follow-up and developed hypothyroidism despite a lack of thyroid antibodies, raising the possibility of cross-reactivity of antibodies to different endocrine glands, or to a more direct cytotoxic effect of ICI therapy. Insulin-dependent diabetes is a rare complication, reported in < 1% of patients treated with ICI, yet GAD antibodies are present in ~20% to 50% of these patients 15,16. Lower PD-L1 expression levels in pancreatic islet cells may lead to autoreactive cytotoxic T-cell-mediated islet destruction 34.
Adrenal, gonadal, and growth hormone levels were not associated with ERAE, but there was a significant association between a decline in TSH from baseline to week 8/9 and from week 8/9 to week 36. This decrease in TSH over time may be predictive of thyroiditis, which commonly precedes the development of central hypothyroidism as an ERAE. A lesser degree of IGF-1 reduction from week 8/9 to week 36 was also associated with the presence of ERAE, but these results should be interpreted with caution given potential confounders. Factors such as weight loss decreased caloric intake, and renal or liver disease can lower IGF-1 levels 35. These factors can fluctuate in advanced cancer, are difficult to control in a study, and therefore the influence of IGF-1 change on ERAE outcomes is debatable.
In 3 patients, antibodies were newly observed at week 8/9, and all 3 individuals developed ERAE. Therefore, evaluation of these makers early in the treatment cycle (ie, prior to ICI treatment and after 3-4 treatment cycles), may allow for early detection and prevention of ERAE with high-grade toxicity.
Patients who developed ERAE had significantly better OS than those without ERAE, consistent with reports of improved survival associated with ICI-induced endocrinopathies 36,37. It is possible that specific ERAE (eg, central hypothyroidism) may have a protective effect on survival, as interestingly, higher TSH levels in older persons are associated with lower mortality rates in the general population 38. However, considering the primary mechanism of action of ICI, a more viable hypothesis is that the presence of ERAE serves as a surrogate biomarker of enhanced ICI efficacy, and is thus reflective of better clearance of tumor cells.
Limitations of this study include the heterogeneity of both cancer and treatment types. The study population included patients with 7 different solid cancer types, although most (>50%) had either melanoma or NSCLC. Six different types of ICI were used, although the majority (75%) were anti-PD-1 therapy (cemiplimab, nivolumab, or pembrolizumab), and the incidence of ERAE is expected to be similar for drugs in this class 5. Given the heterogeneity of cancer types, the improved OS observed in the study should be interpreted with caution, although for other endpoints the heterogeneity of cancer and treatment types in this study may well be reflective of standard clinical settings given the widespread use of ICI for many cancer types.
Conclusion
Prospective evaluation of endocrine-specific autoantibodies showed that the presence of baseline autoantibodies is associated with ERAE pathogenesis and that both baseline and new autoantibody production during ICI treatment may be predictive of ERAE development. To enable prompt diagnosis and treatment of endocrinopathies in patients receiving ICI and help avoid grade 3/4 toxicity, our results support testing for endocrine autoantibodies prior to initiating ICI therapy, and to periodically re-check after treatment is initiated. For those patients who develop such antibodies, more rigorous and frequent endocrine function testing is warranted.
Abbreviations
21-OH
21-hydroxylase
ACTH
adrenocorticotropic hormone
CMIA
chemiluminescent microparticle immunoassay
CTLA-4
cytotoxic T-lymphocyte antigen-4
ERAE
endocrine-related adverse event
Ft4
free thyroxine
FSH
follicle-stimulating hormone
GAD
glutamic acid decarboxylase
IA
immunoassay
ICI
immune checkpoint inhibitor
IGF-1
insulin-like growth factor 1
IQR
interquartile range
irAE
immune-related adverse event
LH
luteinizing hormone
NSCLC
non-small cell lung cancer
OS
overall survival
PD-1
programmed cell death protein 1
PD-L1
programmed death-ligand 1
T3
triiodothyronine
TPO-Ab
thyroid peroxidase antibody
TSH
thyrotropin (thyroid-stimulating hormone)
TSI
thyroid-stimulating immunoglobulin
Acknowledgments
The authors thank Michele Azada and Vivian Hwe for assistance with study coordination, and Shira Berman for assistance with manuscript preparation.
Funding
Supported by CTSI Clinical Scholars Grant (A.L.) under the NIH National Center for Advancing Translational Science (NCATS) University of California Los Angeles Clinical Translational Science Institute (UCLA CTSI) Grant Number UL1TR001881, and by National Institutes of Health (NIH) Grant Number T32DK007770 (S.M.).
Disclosures
O.H. is a consultant/advisory board member for Bristol-Myers Squibb, Genentech, Merck, and Regeneron Pharmaceuticals Inc. A.L., K.W., K.C., L.P., and S.M. have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
1. Wolchok JD, Chan TA. Cancer: antitumor immunity gets a boost. Nature. 2014;515(7528): 496-498.
2. Littman DR. Releasing the brakes on cancer immunotherapy. Cell. 2015;162(6):1186-1190.
3. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management, and surveillance. Nat Rev Clin Oncol. 2019;16(9): 563-580.
4. Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19(1):82-92.
5. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2): 173-182.
6. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypo- physitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99 (11): 4078-4085.
7. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98 (4):1361-1375.
8. Min L, Vaidya A, Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012; 18 (3):351-355.
9. Albarel F, Gaudy C, Castinetti F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172(2): 195-204.
10. Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371-381.
11. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018; 168(2):121-130.
12. Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583-589.
13. Lanzolla G, Coppelli A, Cosottini M, Del Prato S, Marcocci C, Lupi I. Immune checkpoint blockade anti-PD-L1 as a trigger for autoimmune polyendocrine syndrome. J Endocr Soc. 2019;3 (2):496-503.
14. Shi Y, Shen M, Zheng X, et al. ICPis-induced autoimmune polyendocrine syndrome type 2: a review of the literature and a protocol for optimal management. J Clin Endocrinol Metab. 2020; 105(12):e4208-e4218.
15. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018; 103(9): 3144-3154.
16. Tsang VHM, McGrath RT, Clifton-Bligh RJ, et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. 2019;104 (11):5499-5506.
17. Jonklaas J, Bianco AC, Bauer AJ, et al. ; Replacement ATATFoTH. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24 (12):1670-1751.
18. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11): 3888-3921.
19. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364-389.
20. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2021;44 (Suppl 1): S15-S33.
21. Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O’Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010;95 (3):1095-1104.
22. Amino N, Tada H, Hidaka Y, Izumi Y. Postpartum autoimmune thyroid syndrome. Endocr J. 2000;47(6): 645-655.
23. Moleti M, Mauro MD, Alibrandi A, Vita R, Benvenga S, Vermiglio F. Postpartum thyroiditis in women with euthyroid and hypothyroid Hashimoto’s thyroiditis antedating pregnancy. J Clin Endocrinol Metab. 2020;105(7):e2421-e2428.
24. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562.
25. Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2 (2):234-240.
26. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368-376.
27. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36 (19):1905-1912.
28. Tahir SA, Gao J, Miura Y, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci USA. 2019;116(44): 22246-22251.
29. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014; 6(230):230ra 45.
30. Mei Y, Bi WL, Greenwald NF, et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget. 2016;7(47):76565-76576.
31. Percik R, Shlomai G, Tirosh A, et al. Isolated autoimmune adrenocorticotropic hormone deficiency: From a rare disease to the dominant cause of adrenal insufficiency related to checkpoint inhibitors. Autoimmun Rev. 2020;19(2):102454.
32. Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. 2019;181(3): 211-219.
33. Yamamoto M, Iguchi G, Bando H, et al. Autoimmune pituitary disease: new concepts with clinical implications. Endocr Rev. 2020;41 (2):261-272.
34. Yoneda S, Imagawa A, Hosokawa Y, et al. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care. 2019;42(7):e116-e118.
35. Puche JE, Castilla-Cortázar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J Transl Med. 2012;10:224.
36. Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14 (5):e0216954.
37. Kobayashi T, Iwama S, Yasuda Y, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: a prospective study. J ImmunoTher Cancer. 2020; 8(2):e000779.
38. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability, cognitive function, and survival in old age. JAMA. 2004;292(21):2591-2599.
CREDIT: Artak Labadzhyan, Kristopher Wentzel, Omid Hamid, Kamlynn Chow, Sungjin Kim, Lawrence Piro, Shlomo Melmed, Endocrine Autoantibodies Determine Immune Checkpoint Inhibitor- induced Endocrinopathy: A Prospective Study, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 7, July 2022, Pages 1976–1982, https://doi.org/10.1210 /clinem/dgac161