Rachel Rosen
Boston Children’s Hospital, Boston, MA, United States
*Correspondence: Rachel Rosen, ude.dravrah.snerdlihc@nesor.lehcar
Abstract
Gastroesophageal reflux disease has long been implicated as a cause of multiple pediatric symptoms ranging from abdominal pain and regurgitation to cough and dental erosions. Diagnostic testing has evolved greatly over the last 20 years; initial testing with a pH meter to measure esophageal acid reflux burden has evolved into the measurement of both acid and non-acid reflux and liquid and gas reflux. However, measuring reflux burden alone only tells a small part of the GERD story. Many symptoms originally thought to be reflux related are related to other disorders that mimic reflux. The current paradigm involving empiric treatment of symptoms with acid suppression has been replaced with early testing for gastroesophageal reflux and other diagnostic masqueraders. The focus for interventions has shifted away from acid suppression toward motility interventions and includes greater recognition of both functional and motility disorders which present with reflux symptoms.
Keywords:
gastroesophageal reflux disease, impedance, functional luminal impedance planimetry, endoscopy, prucalopride, proton pump inhibitor (PPI)
Tests for Reflux Symptoms
Gastroesophageal reflux has been implicated as a cause of wide-ranging signs and symptoms in children including regurgitation, epigastric pain, cough, and pneumonia. However, studies using pH probes often fail to show a consistent association with reflux events and signs and symptoms. This lack of association raises the questions that: (1) these signs or symptoms are not acid reflux related or that (2) our current technology lacks the sensitivity to measure esophageal events. With the addition of impedance to pH monitoring (pH-MII), clinicians have gained new insight into the role of non-acid reflux and gas in the development of pediatric symptoms; up to 89% of reflux episodes in infants and children are non-acidic and up to 2.4% of symptoms are triggered by gas episodes such as supragastric belching 1–3.
However, beyond just further subtyping reflux categories (acid/ non-acid, liquid/gas/mixed), clues from standard pH-MII testing can help to identify reflux masqueraders. For example, in patients with a high correlation (e.g., >90% of symptoms are associated with reflux events) between reflux events and typical symptoms such as heartburn, regurgitation, and chest pain, rumination syndrome should be considered. While diagnosed by performing standard high-resolution esophageal manometry or 24-h manometry-impedance measurements 4,5, where high-pressure waves are seen emanating from the stomach forcing gastric contents into the stomach, pH-MII clues can support the diagnosis; in addition to a high symptom correlation, a high clustering of symptoms during and immediately following a meal, a high number of full column reflux episodes, a high proportion of symptoms occurring immediately before the esophageal event and a higher prevalence of other functional esophageal disorders (such as supragastric belching which can be seen on the same pH-MII tracings) all support the diagnosis of rumination 6,7. Making a diagnosis of rumination is critical as the therapies are behavioral interventions such as diaphragmatic breathing rather than the addition or escalation of acid suppression therapy. In addition to rumination, a high symptom correlation may also support a diagnosis of reflux hypersensitivity, where patients do not have pathologic amounts of reflux but have a high reflux-symptom correlation; in pediatrics, one-quarter of children have a diagnosis of reflux hypersensitivity 8. While acid suppression may play a role in symptom management, neuromodulators to reduce pain signaling may also be important. While providing evidence of a positive symptom correlation is important to tailor reflux therapies, proving a lack of correlation can be equally important. For example, a diagnosis of functional heartburn is made when patients have typical reflux symptoms but, at the time of the symptoms, there are no reflux events detected by pH or pH-MII monitoring. Since these symptoms are not triggered by reflux events, clinicians can stop prescribing reflux therapies and focus on neuromodulation to address the symptoms.
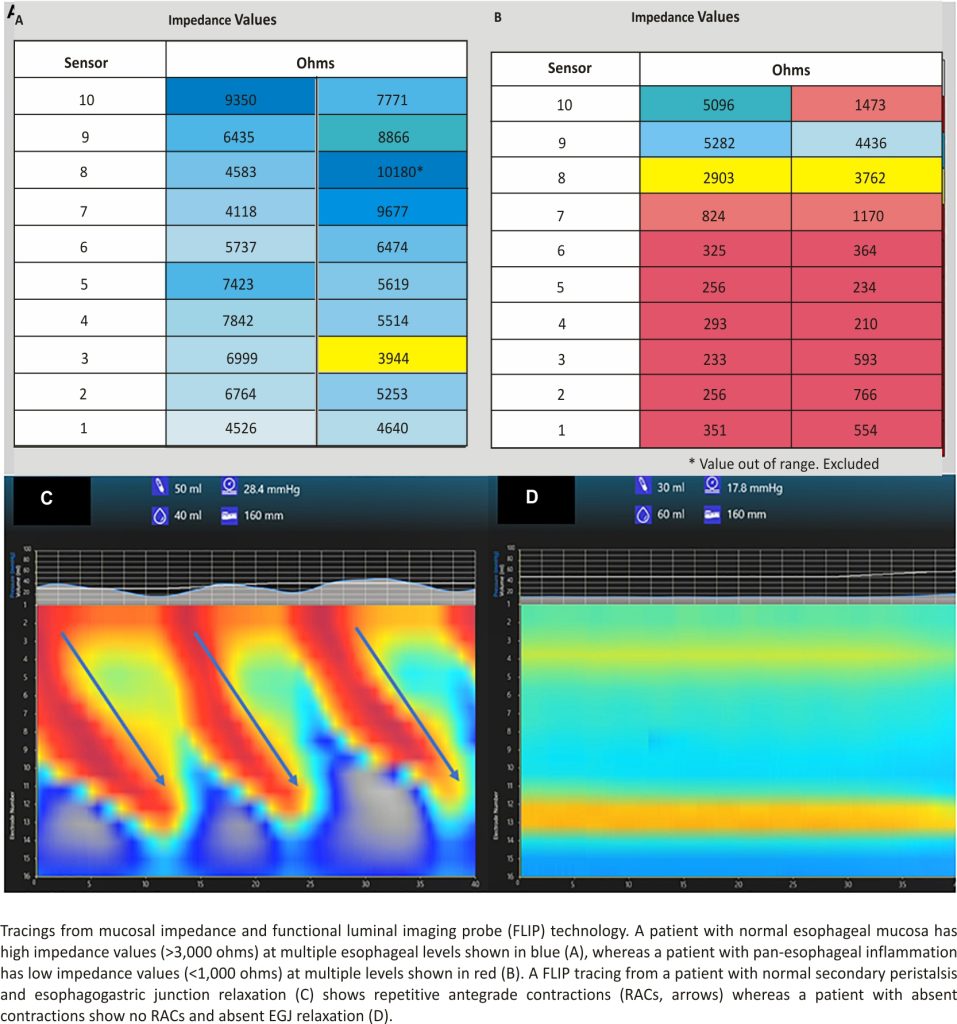
Apart from advances in the interpretation of pH-MII testing, new technologies have provided insight into the risk factors for gastroesophageal reflux and its associated complications. For example, intraprocedural mucosal impedance technology has been used to determine the extent of esophageal inflammation at the time of endoscopy, as a proxy for multiple esophageal biopsies 9–11; while older impedance catheters have been used in pediatric and adult validation studies 10,12, newer technology to more precisely map the esophagus using impedance strips affixed to an esophageal balloon have been used. Depending on the real-time impedance patterns seen when a balloon with impedance strips is inflated in the esophagus to oppose the strips to the mucosa, diagnoses may be made at the time of the index endoscopy and impedance patterns may be followed longitudinally without biopsy; low impedance values signify inflammation and are depicted as a red color and high impedance values signify healthy mucosa and are depicted as a blue color (Figures 1A, B). While not providing different measurements from biopsy in the pediatric population, more precise mapping at multiple esophageal levels might provide some immediate diagnostic clarity with the ultimate goal of reducing pathology costs and reducing the time to a definitive diagnosis. Currently, though, its role in the evaluation of gastroesophageal reflux is not different than multi-level biopsies in children.
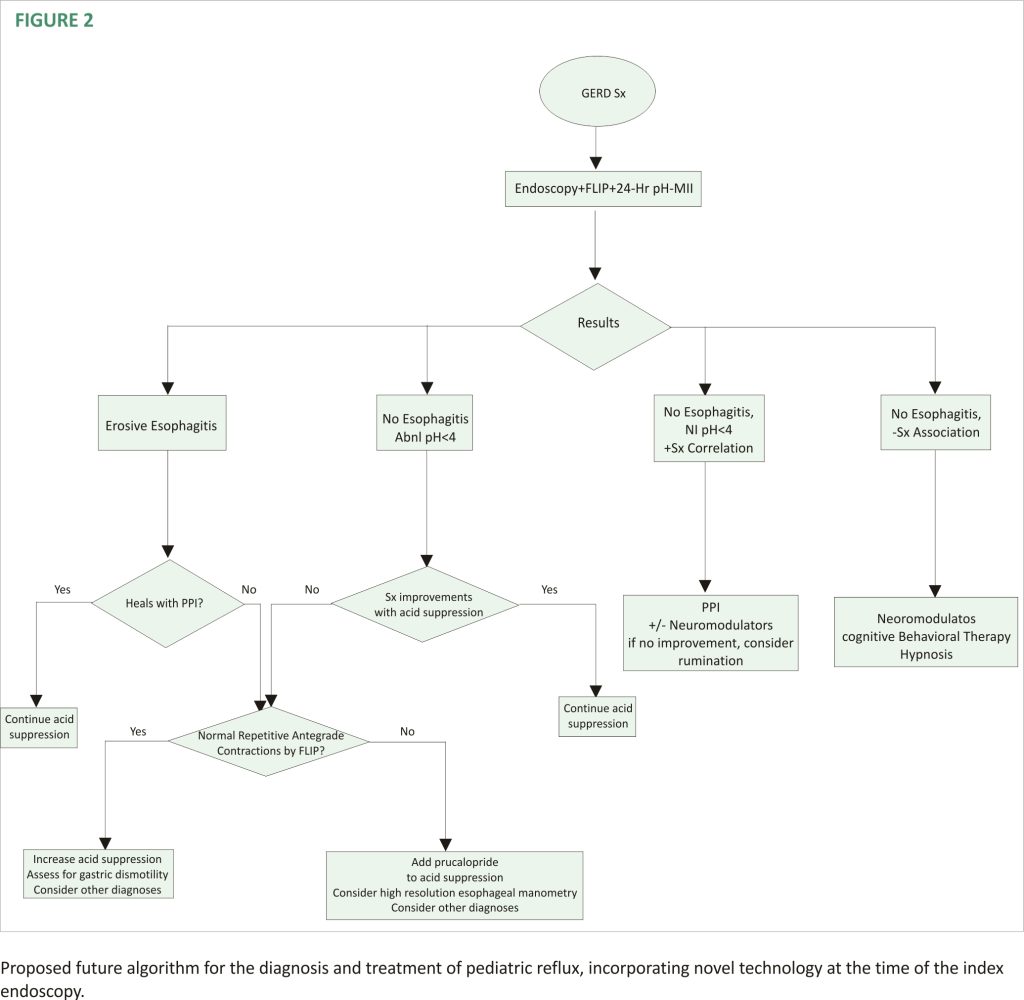
A second new technology that provides insight into the mechanisms of reflux is the functional luminal imaging probe (FLIP). FLIP offers additional insight into esophageal motility and distensibility. By inflating a balloon in the esophagus, sensors can measure (1) how distensible the esophagus and esophagogastric junction (EGJ) are and (2) the strength, frequency, and completeness of esophageal contractions seen with secondary peristalsis. Studies of FLIP in patients at risk for reflux have shown that poor peristaltic response to balloon distension predicts abnormal pH-MII testing and this is independent of EGJ distensibility; intact secondary peristalsis (Figure 1C) is needed to effectively clear reflux episodes 13, a finding corroborated with studies of high-resolution esophageal manometry and pH-MII testing 14. FLIP also has utility in assessing the post-fundoplication esophagus; patients with poor EGJ distensibility or absent secondary peristalsis (Figure 1D) may have a better therapeutic response to interventions such as dilation or lower esophageal BoTox injections 15–17. FLIP holds the greatest potential in revolutionizing the evaluation of pediatric patients with reflux symptoms. The technology, when performed at the time of index endoscopy, screens for both peristaltic disorders and EGJ outflow disorders which can either masquerade as reflux (i.e., retrograde flow of bolus stasis) or can predispose to poor refluxate clearance. This technology is particularly powerful in pediatrics where the performance of esophageal motility studies can be invasive. The potential clinical pathway for pediatric use in the future is shown in Figure 2.
 Paralleling the development of novel technologies which are aimed at improving the diagnosis of reflux and its associated triggers, novel therapies which move away from acid suppression have been developed to treat reflux symptoms. This move away from acid suppression is grounded in evidence that shows an increased risk of complications from acid suppression combined with a lack of efficacy in certain high-risk populations.
Paralleling the development of novel technologies which are aimed at improving the diagnosis of reflux and its associated triggers, novel therapies which move away from acid suppression have been developed to treat reflux symptoms. This move away from acid suppression is grounded in evidence that shows an increased risk of complications from acid suppression combined with a lack of efficacy in certain high-risk populations.
Proton Pump Inhibitor Risk
While proton pump inhibitors (PPIs) are effective in healing esophagitis and improving symptoms in many patients with typical reflux symptoms, new data has emerged about the potential risk. While studies of acid suppression have been shown to alter pediatric and adult infection risk by altering the microbiome, new studies have suggested that viral infection risk may also be increased in adults and children taking PPIs 18–23. Most recently, COVID-19 infection risk and disease severity may be greater in adults and children taking PPIs, a warning to patients with comorbidities that put them at higher risk for more severe disease 24–26. In addition to the newer viral data, studies suggest that PPI use, particularly in early childhood, may increase the risk of the development of eosinophilic esophagitis (EoE) and other allergic diseases potentially converting a short-lived disorder (i.e., GERD) to a lifetime disorder (e.g., EoE) 27,28. Finally, new pediatric data has confirmed what other adult studies have shown, that children exposed to PPIs are at higher risk for bone fractures and this relationship is more significant with cumulative exposure 29,30. Given this new data, thoughtful prescribing (i.e., the lowest dose possible with a regular re-evaluation of need) for acid-related disorders is critical. For patients with persistent symptoms despite acid suppression, early testing and consideration of other therapies are needed.
Medication Therapies Beyond Acid Suppression
Since the only therapy to reduce transient relaxations of the lower esophageal sphincter, the main mechanism of reflux is baclofen which has significant side effects 31–33, the focus of motility therapies has been to improve esophageal and gastric dysmotility based on the hypothesis that improving esophageal motility results in improved reflux clearance and improving gastric motility may reduce the volume of gastric contents that could be refluxed. While early studies have shown an inconsistent relationship between gastric emptying and reflux burden 34–37, recent data have shown that esophageal and gastric dysmotility may be the biggest predictor of persistent reflux symptoms and complications such as esophagitis in adults and select high-risk pediatric populations such as patients with esophageal atresia or with a history of prior lung transplantation 14, 38–40.
One of the mainstays of motility therapy in pediatrics has been erythromycin, a motilin agonist, which increases antral contractions to improve gastric emptying. While early studies using pH probes in preterm infants failed to show a benefit in reducing acid reflux burden in preterm infants, studies were limited because pH-MII was not used so non-acid reflux episodes, the most common type of reflux in preterm infants, could not be detected 41. However, recently a definitive study in preterm infants was performed showing no benefit of erythromycin in reducing total reflux episodes (acid + non-acid) reflux events, a finding similar to that seen in adults using the macrolide azithromycin 42, 43.
Recognizing the disappointing results of macrolides to reduce the total number of gastroesophageal reflux events, other motility agents have been studied. One medication with the most promise because of its effects on both esophageal and gastric motility is prucalopride, a 5-HT4 agonist. While approved as a constipation treatment for adults, the medication has shown significant promise to treat upper tract motility. Placebo-controlled studies of prucalopride in adults have shown that the medication not only increased the peristaltic amplitude but it also increases the number of swallows with complete peristalsis 44, 45. Paralleling the esophageal motility benefits, there are also significant improvements in gastric emptying in healthy controls and patients with delayed gastric emptying 46,47. While these physiology studies have been conducted in adults, there is evidence that prucalopride does improve upper tract symptoms in children; in a study of 71 children, 65% of patients had symptomatic improvement during the follow-up period with the greatest improvements in patients presenting with typical reflux symptoms, vomiting and feeding difficulties 48. This combination of improved esophageal and gastric motility results in reduced acid exposure time in the esophagus, making prucalopride a promising novel therapy for pediatric reflux disease 47.
Another promising reflux therapy for pediatrics is intrapyloric Botulinum toxin (BoTox) injections. While studies have shown that BoTox injections may be beneficial in older children with nausea and vomiting, a study of 112 younger children (mean age: 2.9 ± 1.6 years) with symptoms of reflux, vomiting, and feeding difficulties showed significant symptom improvement with pyloric injections of 6 units/kg 49,50. The response was particularly good in patients with gastroesophageal reflux where 80% of these patients experienced some degree of symptomatic improvement 49. While these results are promising, additional studies are needed to determine the efficacy in patients with isolated GERD and were in the therapeutic algorithm BoTox may fall should larger studies determine if this experimental therapy is efficacious 49.
Another novel medication approach is the use of bile acid sequestrants to reduce the amount of bile reaching the esophagus as bile has been implicated as a cause of esophageal inflammation and, most recently, for more severe extraesophageal symptoms 51,52. In a single adult study of 280 patients with refractory symptoms (defined as persistent symptoms 4 or more times a week despite once daily PPIs), patients were randomized to a placebo or a twice-daily bile acid sequestrant. At high doses of sequestrant, there was a significant improvement in heartburn symptoms over the 8-week trial 53. There are no studies of sequestrants in pediatrics but high concentrations of lung bile portend a worse clinical prognosis suggesting that there may be a role for this class of medications to treat extraesophageal symptoms 51.
Finally, while not novel, neuromodulators have become part of the mainstay of reflux therapy to treat the pain symptoms not responsive to acid suppression (in the case of reflux hypersensitivity) or not associated with reflux events (in the case of functional heartburn). Selective serotonin reuptake inhibitors (e.g., citalopram, fluoxetine), tricyclic antidepressants (e.g., desipramine, imipramine), and GABA analogs (e.g., gabapentin) have been used to modulate both typical and even extraesophageal symptoms and are now part of the algorithms to treat persistent or severe symptoms 54–59. Their role in pediatric reflux is not known.
Lifestyle and Trigger Modification
Dietary modification has long been a part of adult reflux guidelines but there is very little data on the impact of diet on symptomatic control. Mediterranean diets, alkaline water diets, high fiber diets, low fat, low calorie, vegetarian, gluten-free, and low-FODMAP diets have all been studied or proposed in very small trials or in retrospective reviews with varying success in reducing symptoms 60–64. Recent data suggest, however, that clinician-prescribed or self-prescribed diets can result in avoidant-restrictive food intake disorders (ARFID), particularly in adults and children with functional gastrointestinal disorders 65,66. Therefore, with a lack of clear evidence for benefit, the dietary restriction should be recommended very cautiously, particularly in teenagers. In pediatrics, studies of dietary modification have been focused on (1) thickened feeds for infants and children who are enterally fed with either gastrostomy or gastrojejunal feeds; and (2) hypoallergenic formulas for infants. Both thickened feeds and hypoallergenic diets have shown benefits in reducing regurgitation and symptoms attributed to gastroesophageal reflux 67–69.
Another potential modifier for pediatric gastroesophageal reflux is sleep. Studies in adults have suggested that while acid suppression improved nocturnal sleep quality and reduces symptoms of gastroesophageal reflux disease, poor sleep quality can result in pain amplification making reflux symptoms worse 70,71. While there are no comparable pediatric trials, sleep practices have been an integral part of pediatric well-child assessments so represent an easily integrated area of intervention. Complementing regular sleep schedules, and safe sleep positioning has been integral to pediatric good child care, particularly in infants. While supine sleeping is critical for sudden infant death prevention, pediatricians have long known, based on physiology studies, that left lateral positioning reduced gastroesophageal reflux events greater than right lateral positioning so positioning in the awake infant may offer symptom improvement 72,73. Adult studies are just being published confirming the pediatric studies 74.
Conclusion
The field of gastroesophageal reflux in children has changed dramatically with improved testing which has broadened the differential diagnosis to include motility disorders and functional disorders. With these new technologies, more critical information can be obtained at the index endoscopy. This earlier testing results in a faster time-to-definitive diagnosis and allows for more precise tailoring of therapies beyond acid suppression. These diagnostic and therapeutic advances have resulted in a reimaging of existing algorithms including a new proposed pediatric algorithm (Figure 2). However, while the science has advanced significantly for pediatric patients, approval for pediatric medications beyond acid suppression is critically needed to get efficacious medications from the adult realm to the most vulnerable pediatric patients.
Author Contributions
RR conceived of the ideas in this manuscript and wrote the draft in its entirety.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
RR received funding from the NIH R01 Dk097112.
References
1. Halb C, Pomerleau M, Faure C. Multichannel intraesophageal impedance pattern of children with aerophagia. Neurogastroenterol Motil. (2014) 26:1010–4. 10.1111/ nmo.12355
2. Masui D, Nikaki K, Sawada A, Sonmez S, Yazaki E, Sifrim D. Belching in children: prevalence and association with gastroesophageal reflux disease. Neurogastroenterol Motil. (2021) 34:e14194. 10.1111/nmo. 14194
3. Vandenplas Y, Salvatore S, Devreker T, Hauser B. Gastro-oesophageal reflux disease: oesophageal impedance versus pH monitoring. Acta Paediatr. (2007) 96:956–62. 10.1111/j.1651-2227.2007 .00306.x
4. Singendonk MMJ, Ours JM, Bredenoord AJ, Omari TI, van der Pol RJ, Smits MJ, et al. Objectively diagnosing rumination syndrome in children using esophageal pH-impedance and manometry. Neurogastroenterol Motil. (2017) 29:1–8. 10.1111/nmo. 12996
5. Rosen R, Rodriguez L, Nurko S. Pediatric rumination subtypes: a study using high-resolution esophageal manometry with impedance. Neurogastroenterol Motil. (2017) 29:1–9. 10.1111/nmo. 12998
6. Nakagawa K, Sawada A, Hoshikawa Y, Nikaki K, Sonmez S, Woodland P, et al. Persistent postprandial regurgitation vs rumination in patients with refractory gastroesophageal reflux disease symptoms: identification of a distinct rumination pattern using ambulatory impedance-pH monitoring. Am J Gastroenterol. (2019) 114:1248–55? 10.14309/ajg.000000 0000000295
7. Nikaki K, Rybak A, Nakagawa K, Rawat D, Yazaki E, Woodland P, et al. Rumination syndrome in children presenting with refractory gastroesophageal reflux symptoms. J Pediatr Gastroenterol Nutr. (2020) 70:330–5. 10.1097/ MPG.0000 000000002569
8. Mahoney LB, Nurko S, Rosen R. The prevalence of Rome IV nonerosive esophageal phenotypes in children. J Pediatr. (2017) 189:86–91. 10.1016/ j.jpeds.2017.06.019
9. Ates F, Yuksel ES, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. (2015) 148:334 –43.10.1053/j.gastro. 2014.10.010
10. Choksi Y, Lal P, Slaughter JC, Sharda R, Parnell J, Higginbotham T, et al. Esophageal mucosal impedance patterns discriminate patients with eosinophilic esophagitis from patients with GERD. Clin Gastroenterol Hepatol. (2018) 16:664–71.e1. 10.1016/j.cgh. 2017.12.020
11. Kavitt RT, Lal P, Yuksel ES, Ates F, Slaughter JC, Garrett CG, et al. Esophageal mucosal impedance pattern is distinct in patients with extraesophageal reflux symptoms and pathologic acid reflux. J Voice. (2017) 31:347–51. 10.1016/j.jvoice. 2016.06. 023
12. Lowry MA, Vaezi MF, Correa H, Higginbotham T, Slaughter JC, Acra S. Mucosal impedance measurements differentiate pediatric patients with active versus inactive eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2018) 67:198–203. 10.1097/ MPG.0000000000001943
13. Carlson DA, Kathpalia P, Craft J, Tye M, Lin Z, Kahrilas PJ, et al. The relationship between esophageal acid exposure and the esophageal response to volumetric distention. Neurogastroenterol Motil. (2018) 30:1–13. 10.1111/nmo.13240
14. Rogers BD, Rengarajan A, Mauro A, Ghisa M, De Bortoli N, Cicala M, et al. Fragmented and failed swallows on esophageal high-resolution manometry associate with abnormal reflux burden better than weak swallows. Neurogastroenterol Motil. (2020) 32:e13736. 10.1111/nmo.13736
15. Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. (2010) 14:268–76. 10.1007/ s11605-009-1086-1
16. Rosen R, Garza JM, Nurko S. Functional luminal imaging probe assessment in post fundoplication patients changes management beyond manometry. J Pediatr Gastroenterol Nutr. (2020) 70:e119–23. 10.1097/MPG.0000000 000002658
17. Samo S, Mulki R, Godiers ML, Obineme CG, Calderon LF, Bloch JM, et al. Utilizing functional lumen imaging probe in directing treatment for post-fundoplication dysphagia. Surg Endosc. (2021) 35:4418–26. 10.1007/ s00464-020-07941-6
18. Rosen R, Hu L, Amirault J, Khatwa U, Ward DV, Onderdonk A. 16S community profiling identifies proton pump inhibitor-related differences in the gastric, lung, and oropharyngeal microflora. J Pediatr. (2015) 166:917 –23. 10.1016/j.jpeds. 2014.12.067
19. Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. (2006) 117: e817–20. 10.1542/peds.2005-1655
20. Rosen R, Amirault J, Liu H, Mitchell P, Hu L, Khatwa U, et al. Changes in gastric and lung microflora with acid suppression: acid suppression and bacterial growth. JAMA Pediatr. (2014) 168:932–7. 10.1001/JAMA pediatrics.2014.696
21. Prag C, Prag M, Fredlund H. Proton pump inhibitors as a risk factor for norovirus infection. Epidemiol Infect. (2017) 145:1617–23. 10.1017/ S0950268817 000528
22. Vilcu AM, Sabatte L, Blanchon T, Souty C, Maravic M, Lemaitre M, et al. Association between acute gastroenteritis and continuous use of proton pump inhibitors during winter periods of highest circulation of enteric viruses. JAMA Netw Open. (2019) 2:e1916205. 10.1001/ jamanetworkopen.2019.16205
23. Zhou J, Li C, Zhao G, Chu H, Wang D, Yan HH, et al. Human intestinal tract serves as an alternative infection route for middle east respiratory syndrome coronavirus. Sci Adv. (2017) 3:eaao4966. 10.1126/sciadv. aao4966
24. Almario CV, Chey WD, Spiegel BMR. Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol. (2020) 115:1707–15? 10.14309/ajg.0000000000000798
25. Beinvogl B, Cohen A, DiFilippo C, Kane M, Nurko S, Rosen R. Impact of coronavirus disease 2019 on the pediatric population with aerodigestive disease. J Pediatr. (2021) 20:S0022-3476 (21)01228-2. 10.1016/j.jpeds. 2021.12.022
26. Kim HB, Kim JH, Wolf BJ. Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis. Eur J Clin Pharmacol. (2022) 78:383–91. 10.1007/s00228-021-03255-1
27. Kuhn BR, Young AJ, Justice AE, Chittoor G, Walton NA. Infant acid suppression use is associated with the development of eosinophilic esophagitis. Dis Esophagus. (2020) 33:doaa073. 10.1093/dote/ doaa073
28. Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. (2018) 172:e180315. 10.1001/JAMA pediatrics. 2018.0315
29. Fleishman N, Richardson T, Attard T. The clinical characteristics of fractures in pediatric patients exposed to proton pump inhibitors. J Pediatr Gastroenterol Nutr. (2020) 70:815–9. 10.1097/MPG. 0000000000002690
30. Wang YH, Wintzell V, Ludvigsson JF, Svanstrom H, Pasternak B. Association between proton pump inhibitor use and risk of fracture in children. JAMA Pediatr. (2020) 174: 543–51. 10.1001/JAMA pediatrics. 2020.0007
31. Ewer AK, Durbin GM, Morgan ME, Booth IW. Gastric emptying and gastro-oesophageal reflux in preterm infants. Arch Dis Child Fetal Neonatal Ed. (1996) 75:F117–21. 10.1136/ fn.75.2.f117
32. Knatten CK, Avitsland TL, Medhus AW, Fjeld JG, Pripp AH, Emblem R, et al. Gastric emptying in children with gastroesophageal reflux and healthy children. J Pediatr Surg. (2013) 48:1856–61. 10.1016/j.j pedsurg.2013. 03.076
33. Machado RS, Yamamoto E, da Silva Patricio FR, Reber M, Kawakami E. Gastric emptying evaluation in children with erosive gastroesophageal reflux disease. Pediatr Surg Int. (2010) 26:473–8. 10.1007/s00383-010-2579-4
34. Papaila JG, Wilmot D, Grosfeld JL, Rescorla FJ, West KW, Vane DW. Increased incidence of delayed gastric emptying in children with gastroesophageal reflux. A prospective evaluation. Arch Surg. (1989) 124:933–6. 10.1001/archsurg. 1989. 01410080065010
35. Yasuda JL, Staffa SJ, Nurko S, Kane M, Wall S, Mougey EB, et al. Pharmacogenomics fails to explain proton pump inhibitor refractory esophagitis in pediatric esophageal atresia. Neurogastroenterol Motil. (2021) 34:e14217. 10.1111/nmo. 14217
36. Jamie Dy F, Freiberger D, Liu E, Boyer D, Visner G, Rosen R. Impact of gastroesophageal reflux and delayed gastric emptying on pediatric lung transplant outcomes. J Heart Lung Transplant. (2017) 36:854–61. 10.1016/j.healun.2017.01.005
37. Lotstedt B, Boyer D, Visner G, Freiberger D, Lurie M, Kane M, et al. The impact of gastrointestinal dysmotility on the aerodigestive microbiome of pediatric lung transplant recipients. J Heart Lung Transplant. (2020) 40:210–9. 10.1016/j.healun. 2020.11.013
38. Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. (2015) 80:200–8. 10.1111 /BCP.12619
39. Kawai M, Kawahara H, Hirayama S, Yoshimura N, Ida S. Effect of baclofen on emesis and 24-hour esophageal pH in neurologically impaired children with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. (2004) 38:317–23. 10.1097/000051 76-20040 3000-00017
40. Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. J Pediatr. (2006) 149: 468–74. 10.1016/j.jpeds.2006. 05.029
41. Ng SC, Gomez JM, Rajadurai VS, Saw SM, Quak SH. Establishing enteral feeding in preterm infants with feeding intolerance: a randomized controlled study of low-dose erythromycin. J Pediatr Gastroenterol Nutr. (2003) 37:554–8. 10.1097/00005176-200311000-00009
42. Rohof WO, Bennink RJ, de Ruigh AA, Hirsch DP, Zwinderman AH, Boeckxstaens GE. Effect of azithromycin on acid reflux, hiatus hernia, and proximal acid pocket in the postprandial period. Gut. (2012) 61:1670–7. 10.1136/gut jnl-2011-300926
43. Ballengee CR, Davalian F, Conaway MR, Sauer CG, Kaufman DA. Erythromycin and reflux events in premature neonates: a randomized clinical trial. J Pediatr Gastroenterol Nutr. (2018) 67:720–5. 10.1097/MPG.0000000 000002086
44. Lei WY, Hung JS, Liu TT, Yi CH, Chen CL. Influence of prucalopride on esophageal secondary peristalsis in reflux patients with ineffective motility. J Gastroenterol Hepatol. (2018) 33:650–5. 10.1111/ jgh.13986
45. Yi CH, Lei WY, Hung JS, Liu TT, Chen CL. Effects of prucalopride on esophageal secondary peristalsis in humans. Clin Transl Gastroenterol. (2016) 7:e202. 10.1038/ctg.2016.58
46. Carbone F, Van den Houte K, Clevers E, Andrews CN, Papathanasopoulos A, Holvoet L, et al. Prucalopride in gastroparesis: a randomized placebo-controlled crossover study. Am J Gastroenterol. (2019) 114:1265–74? 10.14309/ajg.0000000000000304
47. Kessing BF, Smout AJ, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. (2014) 26:1079–86. 10.1111/ nmo.12359
48. Hirsch S, Nurko S, Mitchell P, Rosen R. Prucalopride for treatment of upper gastrointestinal symptoms in children. Paediatr Drugs. (2022) 24:73–81. 10. 1007/s40272-021-00489-5
49. Hirsch S, Nurko S, Mitchell P, Rosen R. Botulinum toxin as a treatment for feeding difficulties in young children. J Pediatr. (2020) 226:228–35. 10.1016 /j.jpeds.2020.06.063
50. Rodriguez L, Rosen R, Manfredi M, Nurko S. Endoscopic intrapyloric injection of botulinum toxin A in the treatment of children with gastroparesis: a retrospective, open-label study. Gastrointest Endosc. (2012) 75:302 –9. 10.1016/j.gie.2011.09.042
51. Rosen R, Lurie M, Kane M, DiFilippo C, Cohen A, Freiberger D, et al. Risk factors for bile aspiration and its impact on clinical outcomes. Clin Transl Gastroenterol. (2021) 12:e004 34. 10.14309/ctg.00000000000 00434
52. Song S, Guha S, Liu K, Buttar NS, Bresalier RS. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signaling pathways in Barrett’s esophagus and oesophageal adenocarcinoma. Gut. (2007) 56:1512–21. 10.1136/gut.2007.121 244
53. Vaezi MF, Fass R, Vakil N, Reasner DS, Mittleman RS, Hall M, et al. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a randomized trial. Gastroenterology. (2020) 158: 2093–103. 10.1053/j.gastro.2020. 02.031
54. Viazis N, Keyoglou A, Kanellopoulos AK, Karamanolis G, Vlachogiannakos J, Triantafyllou K, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. (2012) 107:1662–7? 10.1038/ajg. 2011.179
55. Ostovaneh MR, Saeidi B, Hajifathalian K, Farrokhi-Khajeh-Pasha Y, Fotouhi A, Mirbagheri SS, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: a double-blind placebo-controlled trial. Neurogastroenterol Motil. (2014) 26:670–8. 10.1111/nmo.12 313
56. Spechler SJ, Hunter JG, Jones KM, Lee R, Smith BR, Mashimo H, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. (2019) 381:1513–23. 10.1056/NEJMoa1 811424
57. Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, Leelakusolvong S. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol. (2016) 111:217–24? 10.1038/ajg.2015.413
58. Dong R, Xu X, Yu L, Ding H, Pan J, Yu Y, et al. Randomised clinical trial: gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough. Aliment Pharmacol Ther. (2019) 49:714–22. 10.1111/apt. 15169
59. Zhang M, Zhu Y, Dong R, Qiu Z. Gabapentin versus baclofen for the treatment of refractory gastroesophageal reflux-induced chronic cough. J Thorac Dis. (2020) 12:5243–50. 10.21037/jtd-2020-icc-002
60. Zalvan CH, Hu S, Greenberg B, Geliebter J. A comparison of alkaline water and Mediterranean diet vs proton pump inhibition for the treatment of laryngopharyngeal reflux. JAMA Otolaryngol Head Neck Surg. (2017) 143:1023–9. 10.1001/jamaoto. 2017. 1454
61. Geysen H, Gielis E, Deloose E, Vanuytsel T, Tack J, Biesiekierski JR, et al. Acute administration of fructans increases the number of transient lower esophageal sphincter relaxations in healthy volunteers. Neurogastroenterol Motil. (2020) 32:e13727. 10.1111/nmo .13727
62. Zanini B, Basche R, Ferraresi A, Ricci C, Lanzarotto F, Marullo M, et al. Randomised clinical study: gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment Pharmacol Ther. (2015) 42:968–76. 10.1111/apt. 13372
63. Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol. (2018) 24: 2291–9. 10.3748/wjg.v24.i21. 2291
64. Tosetti C, Savarino E, Benedetto E, De Bastiani R. Study Group for the Evaluation of Gerd Triggering Foods. Elimination of dietary triggers is successful in treating symptoms of gastroesophageal reflux disease. Dig Dis Sci. (2021) 66:1565–71. 10.1007/ s10620-020-06414-z
65. Murray HB, Rao FU, Baker C, Silvernale CJ, Staller K, Harshman SG, et al. Prevalence and characteristics of avoidant/restrictive food intake disorder in pediatric neurogastroenterology patients. J Pediatr Gastroenterol Nutr. (2021). 10.1097/MPG.00000000000 03369
66. Murray HB, Kuo B, Eddy KT, Breithaupt L, Becker KR, Dreier MJ, et al. Disorders of gut-brain interaction common among outpatients with eating disorders including avoidant/ restrictive food intake disorder. Int J Eat Disord. (2021) 54:952–8. 10. 1002/eat.23414
67. Hron B, Fishman E, Lurie M, Clarke T, Chin Z, Hester L, et al. health outcomes and quality of life indices of children receiving blenderized feeds via enteral tube. J Pediatr. (2019) 211:139–45.e1.
68. Borrelli O, Mancini V, Thapar N, Giorgio V, Elawad M, Hill S, et al. Cow’s milk challenge increases weakly acidic reflux in children with cow’s milk allergy and gastroesophageal reflux disease. J Pediatr. (2012) 161:476– 81.e1. 10.1016 /j.jpeds.2012.03.002
69. Omari T, Tobin JM, McCall L, Savage K, Ferris L, Hammond P, et al. Characterization of upper gastrointestinal motility in infants with persistent distress and non-IgE-mediated cow’s milk protein allergy. J Pediatr Gastroenterol Nutr. (2020) 70:489 –96. 10.1097/MPG.0000000000 002600
70. Schey R, Dickman R, Parthasarathy S, Quan SF, Wendel C, Merchant J, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology. (2007) 133:1787–95. 10.1053/j. gastro.2007. 09.039
71. Johnson DA, Orr WC, Crawley JA, Traxler B, McCullough J, Brown KA, et al. Effect of esomeprazole on nighttime heartburn and sleep quality in patients with GERD: a randomized, placebo-controlled trial. Am J Gastroenterol. (2005) 100: 1914–22. 10.1111/j.1572-0241. 2005 .00285.x
72. Loots C, Kritas S, van Wijk M, McCall L, Peeters L, Lewindon P, et al. Body positioning and medical therapy for infantile gastroesophageal reflux symptoms. J Pediatr Gastroenterol Nutr. (2014) 59:237–43. 10.1097/ MPG. 0000000000000395
73. Loots C, Smits M, Omari T, Bennink R, Benninga M, van Wijk M. Effect of lateral positioning on gastroesophageal reflux (GER) and underlying mechanisms in GER disease (GERD) patients and healthy controls. Neurogastroenterol Motil. (2013) 25:222–9; e161–2. 10.1111 /nmo.12042
74. Schuitenmaker JM, van Dijk M, Oude Nijhuis RAB, Smout A, Bredenoord AJ. Associations between sleep position and nocturnal gastroesophageal reflux: a study using concurrent monitoring of sleep position and esophageal pH and impedance. Am J Gastroenterol. (2022) 117:346–51? 10.14309/ajg.00000 00000001588
CREDITS: Rosen R. Novel Advances in the Evaluation and Treatment of Children With Symptoms of Gastroesophageal Reflux Disease. Front Pediatr. 2022 Apr 1;10: 849105. doi: 10.3389/fped. 2022.849105. PMID: 35433543; PMCID: PMC9010502.